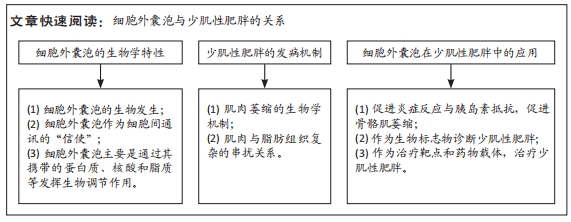
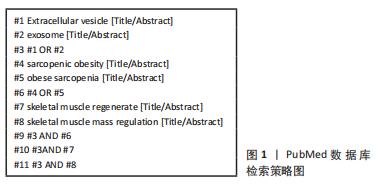
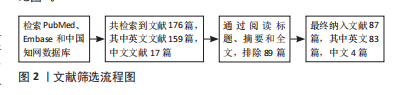
[1] CRUZ-JENTOFT AJ, SAYER AA. Sarcopenia. Lancet. 2019;393(10191):2636-2646.
[2] GAO Q, MEI F, SHANG Y, et al. Global prevalence of sarcopenic obesity in older adults: a systematic review and meta-analysis. Clin Nutr. 2021;40(7):4633-4641.
[3] 吉彤,汤哲, 李耘,等.老年人少肌性肥胖预防和治疗策略 [J].中华老年医学杂志,2020,39(7):845-849.
[4] ROUBENOFF R. Sarcopenic obesity: the confluence of two epidemics. Obes Res. 2004;12(6):887-888.
[5] STEWART ST, CUTLER DM, ROSEN AB. Forecasting the effects of obesity and smoking on U.S. life expectancy. N Engl J Med. 2009;361(23):2252-2260.
[6] KIM Y, WHITE T, WIJNDAELE K, et al. Adiposity and grip strength as long-term predictors of objectively measured physical activity in 93 015 adults: the UK Biobank study. Int J Obes (Lond). 2017;41(9):1361-1368.
[7] MA J, HWANG SJ, MCMAHON GM, et al. Mid-adulthood cardiometabolic risk factor profiles of sarcopenic obesity. Obesity (Silver Spring). 2016;24(2): 526-534
[8] AKBAR N, DIGBY JE, CAHILL TJ, et al. Endothelium-derived extracellular vesicles promote splenic monocyte mobilization in myocardial infarction. JCI Insight. 2017;2(17):e93344.
[9] LIU ML, WERTH VP, WILLIAMS KJ. Blood plasma versus serum: which is right for sampling circulating membrane microvesicles in human subjects? Ann Rheum Dis. 2020;79(6):e73.
[10] 王琎,陈建英. 细胞外囊泡研究新进展[J].中国组织工程研究,2017, 21(4):621-626.
[11] CYPESS AM, LEHMAN S, WILLIAMS G, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360(15):1509-1517.
[12] LEHMANN BD, PAINE MS, BROOKS AM, et al. Senescence-associated exosome release from human prostate cancer cells. Cancer Res. 2008;68(19):7864-7871.
[13] ROME S. Muscle and adipose tissue communicate with extracellular vesicles. Int J Mol Sci. 2022;23(13):7052.
[14] 杨东丽,杨琼,罗嘉,等.外泌体参与骨骼肌机能调控研究进展[J].动物医学进展,2018,39(1):103-108.
[15] STEPANIAN A, BOURGUIGNAT L, HENNOU S, et al. Microparticle increase in severe obesity: not related to metabolic syndrome and unchanged after massive weight loss. Obesity (Silver Spring). 2013;21(11):2236-2243.
[16] EGUCHI A, LAZIC M, ARMANDO AM, et al. Circulating adipocyte-derived extracellular vesicles are novel markers of metabolic stress. J Mol Med (Berl). 2016;94(11):1241-1253.
[17] CHEN Y, LI G, LIU ML. Microvesicles as emerging biomarkers and therapeutic targets in cardiometabolic diseases. Genomics Proteomics Bioinformatics. 2018;16(1):50-62.
[18] BI P, MCANALLY JR, SHELTON JM, et al. Fusogenic micropeptide Myomixer is essential for satellite cell fusion and muscle regeneration. Proc Natl Acad Sci U S A. 2018;115(15):3864-3869.
[19] TIDBALL J G. Inflammatory processes in muscle injury and repair. Am J Physiol Regul Integr Comp Physiol. 2005;288(2):R345-R353.
[20] DEMONBREUN AR, MCNALLY EM. Muscle cell communication in development and repair. Curr Opin Pharmacol. 2017;34:7-14.
[21] JOANISSE S, NEDERVEEN JP, SNIJDERS T, et al. Skeletal muscle regeneration, repair and remodelling in aging: the importance of muscle stem cells and vascularization. Gerontology. 2017;63(1):91-100.
[22] WOLF P. The nature and significance of platelet products in human plasma. Br J Haematol. 1967;13(3):269-288.
[23] AKERS JC, GONDA D, KIM R, et al. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neurooncol. 2013;113(1):1-11.
[24] ZIJLSTRA A, DI VIZIO D. Size matters in nanoscale communication. Nat Cell Biol. 2018;20(3):228-230.
[25] MAAS SLN, BREAKEFIELD XO, WEAVER AM. Extracellular vesicles: unique intercellular delivery vehicles. Trends Cell Biol. 2017;27(3):172-188.
[26] VAN NIEL G, CHARRIN S, SIMOES S, et al. The tetraspanin CD63 regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis. Dev Cell. 2011;21(4):708-721.
[27] LOBB RJ, BECKER M, WEN SW, et al. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J Extracell Vesicles. 2015;4: 27031.
[28] NIU C, WANG X, ZHAO M, et al. Macrophage foam cell-derived extracellular vesicles promote vascular smooth muscle cell migration and adhesion. J Am Heart Assoc. 2016;5(10):e004099.
[29] HUANG Z, XU A. Adipose Extracellular vesicles in intercellular and inter-organ crosstalk in metabolic health and diseases. Front Immunol. 2021;12: 608680.
[30] KALRA H, DRUMMEN GPC, MATHIVANAN S. Focus on extracellular vesicles: introducing the next small big thing. Int J Mol Sci. 2016;17(2):170.
[31] MINCIACCHI VR, FREEMAN MR, DI VIZIO D. Extracellular vesicles in cancer: exosomes, microvesicles and the emerging role of large oncosomes. Semin Cell Dev Biol. 2015;40:41-51.
[32] BATSIS JA, VILLAREAL DT. Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol. 2018;14(9): 513-537.
[33] SHAO X, GONG W, WANG Q, et al. Atrophic skeletal muscle fibre-derived small extracellular vesicle miR-690 inhibits satellite cell differentiation during ageing. J Cachexia Sarcopenia Muscle. 2022;13(6):3163-3180.
[34] ITOKAZU M, ONODERA Y, MORI T, et al. Adipose-derived exosomes block muscular stem cell proliferation in aged mouse by delivering miRNA Let-7d-3p that targets transcription factor HMGA2. J Biol Chem. 2022; 298(7):102098.
[35] VALADI H, EKSTRöM K, BOSSIOS A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654-659.
[36] DALLE S, ROSSMEISLOVA L, KOPPO K. The Role of Inflammation in Age-Related Sarcopenia. Front Physiol. 2017;8:1045.
[37] IDOATE F, CADORE EL, CASAS-HERRERO A, et al. Adipose tissue compartments, muscle mass, muscle fat infiltration, and coronary calcium in institutionalized frail nonagenarians. Eur Radiol. 2015;25(7):2163-2175.
[38] PORTER C, HURREN NM, COTTER MV, et al. Mitochondrial respiratory capacity and coupling control decline with age in human skeletal muscle. Am J Physiol Endocrinol Metab. 2015;309(3):E224-E232.
[39] FERRANTE SC, NADLER EP, PILLAI DK, et al. Adipocyte-derived exosomal miRNAs: a novel mechanism for obesity-related disease. Pediatr Res. 2015;77(3):447-454.
[40] HUANG-DORAN I, ZHANG CY, VIDAL-PUIG A. Extracellular vesicles: novel mediators of cell communication in metabolic disease. Trends Endocrinol Metab. 2017;28(1):3-18.
[41] WANNAMETHEE SG, ATKINS JL. Muscle loss and obesity: the health implications of sarcopenia and sarcopenic obesity. Proc Nutr Soc. 2015; 74(4):405-412.
[42] KALINKOVICH A, LIVSHITS G. Sarcopenic obesity or obese sarcopenia: a cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res Rev. 2017;35:200-221.
[43] GAMBACCIANI M, CIAPONI M, CAPPAGLI B, et al. Prospective evaluation of body weight and body fat distribution in early postmenopausal women with and without hormonal replacement therapy. Maturitas. 2001;39(2):125-132.
[44] KADI F. Cellular and molecular mechanisms responsible for the action of testosterone on human skeletal muscle. A basis for illegal performance enhancement. Br J Pharmacol. 2008;154(3):522-528.
[45] NILWIK R, SNIJDERS T, LEENDERS M, et al. The decline in skeletal muscle mass with aging is mainly attributed to a reduction in type II muscle fiber size. Exp Gerontol. 2013;48(5):492-498.
[46] VERDIJK LB, SNIJDERS T, DROST M, et al. Satellite cells in human skeletal muscle; from birth to old age. Age (Dordr). 2014;36(2):545-547.
[47] CAMINO T, LAGO-BAAMEIRO N, BRAVO SB, et al. Human obese white adipose tissue sheds depot-specific extracellular vesicles and reveals candidate biomarkers for monitoring obesity and its comorbidities. Transl Res. 2022;239:85-102.
[48] MONTECALVO A, LARREGINA AT, SHUFESKY WJ, et al. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood. 2012;119(3):756-766.
[49] FRüHBECK G, GóMEZ-AMBROSI J, MURUZáBAL FJ, et al. The adipocyte: a model for integration of endocrine and metabolic signaling in energy metabolism regulation. Am J Physiol Endocrinol Metab. 2001;280(6):E827-E847.
[50] HUBAL MJ, NADLER EP, FERRANTE SC, et al. Circulating adipocyte-derived exosomal MicroRNAs associated with decreased insulin resistance after gastric bypass. Obesity (Silver Spring). 2017;25(1):102-110.
[51] VECHETTI IJ, PECK BD, WEN Y, et al. Mechanical overload-induced muscle-derived extracellular vesicles promote adipose tissue lipolysis. FASEB J. 2021;35(6):e21644.
[52] DE GASPERI R, HAMIDI S, HARLOW LM, et al. Denervation-related alterations and biological activity of miRNAs contained in exosomes released by skeletal muscle fibers. Sci Rep. 2017;7(1):12888.
[53] HUANG D, CHEN J, HU D, et al. Advances in biological function and clinical application of small extracellular vesicle membrane proteins. Front Oncol. 2021;11:675940.
[54] OUCHI N, PARKER JL, LUGUS JJ, et al. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11(2):85-97.
[55] CAMPELLO E, ZABEO E, RADU C M, et al. Dynamics of circulating microparticles in obesity after weight loss. Intern Emerg Med. 2016;11(5):695-702.
[56] SONG HJ, OH S, QUAN S, et al. Gender differences in adiponectin levels and body composition in older adults: Hallym aging study. BMC Geriatr. 2014;14:8.
[57] CAN B, KARA O, KIZILARSLANOGLU MC, et al. Serum markers of inflammation and oxidative stress in sarcopenia. Aging Clin Exp Res. 2017;29(4):745-752.
[58] WANG YC, LI Y, WANG XY, et al. Circulating miR-130b mediates metabolic crosstalk between fat and muscle in overweight/obesity. Diabetologia. 2013;56(10):2275-2285.
[59] 付婉瑞,古再丽努尔·卡德尔,冯颖.增龄相关肌少性肥胖的生物信息学分析[J].老年医学与保健,2022,28(2):285-290,295.
[60] YIN X, TIAN M, ZHANG J, et al. MiR-26b-5p in small extracellular vesicles derived from dying tumor cells after irradiation enhances the metastasis promoting microenvironment in esophageal squamous cell carcinoma. Cancer Lett. 2022; 541:215746.
[61] ZHAO J, LIN H, HUANG K, et al. Cancer-associated fibroblasts-derived extracellular vesicles carrying lncRNA SNHG3 facilitate colorectal cancer cell proliferation via the miR-34b-5p/HuR/HOXC6 axis. Cell Death Discov. 2022;8(1):346.
[62] TAAFFE DR, DURET C, WHEELER S, et al. Once-weekly resistance exercise improves muscle strength and neuromuscular performance in older adults. J Am Geriatr Soc. 1999;47(10):1208-1214.
[63] PEDERSEN BK, SALTIN B. Exercise as medicine -evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports. 2015;25 Suppl 3:1-72.
[64] NAIR VD, GE Y, LI S, et al. Sedentary and trained older men have distinct circulating exosomal microRNA profiles at baseline and in response to acute exercise. Front Physiol. 2020;11:605.
[65] WHITHAM M, PARKER BL, FRIEDRICHSEN M, et al. Extracellular vesicles provide a means for tissue crosstalk during exercise. Cell Metab. 2018;27(1):237-251.e4.
[66] WIKLANDER OPB, BRENNAN MÁ, LöTVALL J, et al. Advances in therapeutic applications of extracellular vesicles Sci Transl Med. 2019;11(492):eaav8521.
[67] EL ANDALOUSSI S, MäGER I, BREAKEFIELD XO, et al. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12(5):347-357.
[68] FREEMAN DW, NOREN HOOTEN N, EITAN E, et al. Altered extracellular vesicle concentration, cargo, and function in diabetes. Diabetes. 2018; 67(11):2377-2388.
[69] CHOI Y, KWON Y, KIM DK, et al. Gut microbe-derived extracellular vesicles induce insulin resistance, thereby impairing glucose metabolism in skeletal muscle. Sci Rep. 2015;5:15878.
[70] KUMAR A, SUNDARAM K, MU J, et al. High-fat diet-induced upregulation of exosomal phosphatidylcholine contributes to insulin resistance. Nat Commun. 2021;12(1):213.
[71] O’LEARY MF, WALLACE GR, BENNETT AJ, et al. IL-15 promotes human myogenesis and mitigates the detrimental effects of TNFα on myotube development. Sci Rep. 2017;7(1):12997.
[72] YALCIN A, SILAY K, BALIK AR, et al. The relationship between plasma interleukin-15 levels and sarcopenia in outpatient older people. Aging Clin Exp Res. 2018;30(7):783-790.
[73] WATERS DL, QUALLS CR, DORIN RI, et al. Altered growth hormone, cortisol, and leptin secretion in healthy elderly persons with sarcopenia and mixed body composition phenotypes. J Gerontol A Biol Sci Med Sci. 2008;63(5):536-541.
[74] KOHARA K, OCHI M, TABARA Y, et al. Leptin in sarcopenic visceral obesity: possible link between adipocytes and myocytes. PLoS One. 2011;6(9):e24633.
[75] REBALKA IA, MONACO CMF, VARAH NE, et al. Loss of the adipokine lipocalin-2 impairs satellite cell activation and skeletal muscle regeneration. Am J Physiol Cell Physiol. 2018;315(5):C714-C721.
[76] SANDRI M, BARBERI L, BIJLSMA AY, et al. Signalling pathways regulating muscle mass in ageing skeletal muscle: the role of the IGF1-Akt-mTOR-FoxO pathway. Biogerontology. 2013;14(3):303-323.
[77] ZHANG L, ZHANG D, QIN ZY, et al. The role and possible mechanism of long noncoding RNA PVT1 in modulating 3T3-L1 preadipocyte proliferation and differentiation. IUBMB Life. 2020;72(7):1460-1467.
[78] ZHAO Y, ZHAO J, GUO X, et al. Long non-coding RNA PVT1, a molecular sponge for miR-149, contributes aberrant metabolic dysfunction and inflammation in IL-1β-simulated osteoarthritic chondrocytes. Biosci Rep. 2018;38(5):BSR20180576.
[79] CHEN JF, MANDEL EM, THOMSON JM, et al. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet. 2006;38(2):228-233.
[80] NAKASA T, ISHIKAWA M, SHI M, et al. Acceleration of muscle regeneration by local injection of muscle-specific microRNAs in rat skeletal muscle injury model. J Cell Mol Med. 2010;14(10):2495-2505.
[81] DRUMMOND MJ, MCCARTHY JJ, SINHA M, et al. Aging and microRNA expression in human skeletal muscle: a microarray and bioinformatics analysis. Physiol Genomics. 2011;43(10):595-603.
[82] DRUMMOND MJ, MCCARTHY JJ, FRY CS, et al. Aging differentially affects human skeletal muscle microRNA expression at rest and after an anabolic stimulus of resistance exercise and essential amino acids. Am J Physiol Endocrinol Metab. 2008;295(6):E1333-E1340.
[83] LI YJ, WU JY, WANG JM, et al. Emerging strategies for labeling and tracking of extracellular vesicles. J Control Release. 2020;328:141-159.
[84] HUANG Y, ZHU X, CHEN K, et al. Resveratrol prevents sarcopenic obesity by reversing mitochondrial dysfunction and oxidative stress via the PKA/LKB1/AMPK pathway. Aging (Albany NY). 2019;11(8):2217-2240.
[85] IGLESIAS-AGUIRRE CE, ÁVILA-GáLVEZ MÁ, LóPEZ DE LAS HAZAS MC, et al. Exosome-containing extracellular vesicles contribute to the transport of resveratrol metabolites in the bloodstream: a human pharmacokinetic study. Nutrients. 2022;14(17):3632.
[86] MASTROTOTARO L, RODEN M. Insulin resistance and insulin sensitizing agents. Metabolism. 2021;125:154892.
[87] FIGLIOLINI F, RANGHINO A, GRANGE C, et al. Extracellular vesicles from adipose stem cells prevent muscle damage and inflammation in a mouse model of hind limb ischemia: role of neuregulin-1. Arterioscler Thromb Vasc Biol. 2020;40(1):239-254. |