中国组织工程研究 ›› 2024, Vol. 28 ›› Issue (1): 113-120.doi: 10.12307/2023.921
• 干细胞综述 stem cell review • 上一篇 下一篇
类装配体的构建方法及应用
刘春磊1,2,3,姚 茜1,3,韦正波2,3,谢 莹1,3
- 1广西医科大学生命科学研究院,广西壮族自治区南宁市 530021;2广西医科大学附属肿瘤医院,广西壮族自治区南宁市 530021;3广西区域性高发肿瘤早期防治研究重点实验室,广西壮族自治区南宁市 530021
-
收稿日期:2023-01-06接受日期:2023-02-18出版日期:2024-01-08发布日期:2023-06-29 -
通讯作者:谢莹,博士,教授,广西医科大学生命科学研究院,广西壮族自治区南宁市 530021;广西区域性高发肿瘤早期防治研究重点实验室,广西壮族自治区南宁市 530021 -
作者简介:刘春磊,男,1991年生,河南省周口市人,汉族,广西医科大学在读硕士,主要从事类器官相关研究。 姚茜,女,1989年生,湖北省人,蒙古族,2019年广西医科大学毕业,硕士,主要从事类器官相关研究。 -
基金资助:国家自然科学基金项目 (82160386),项目负责人:谢莹;广西自然科学基金面上项目(2021GXNSFAA075042),项目负责人:谢莹
Construction methods and application of assembloids
Liu Chunlei1, 2, 3, Yao Xi1, 3, Wei Zhengbo2, 3, Xie Ying1, 3
- 1Life Science Institute, Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China; 2Affiliated Tumor Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China; 3Guangxi Key Laboratory of Early Prevention and Treatment for Regional High Frequency Tumor, Nanning 530021, Guangxi Zhuang Autonomous Region, China
-
Received:2023-01-06Accepted:2023-02-18Online:2024-01-08Published:2023-06-29 -
Contact:Xie Ying, PhD, Professor, Life Science Institute, Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China; Guangxi Key Laboratory of Early Prevention and Treatment for Regional High Frequency Tumor, Nanning 530021, Guangxi Zhuang Autonomous Region, China -
About author:Liu Chunlei, Master candidate, Life Science Institute, Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China; Affiliated Tumor Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China; Guangxi Key Laboratory of Early Prevention and Treatment for Regional High Frequency Tumor, Nanning 530021, Guangxi Zhuang Autonomous Region, China Yao Xi, Master, Life Science Institute, Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China; Guangxi Key Laboratory of Early Prevention and Treatment for Regional High Frequency Tumor, Nanning 530021, Guangxi Zhuang Autonomous Region, China -
Supported by:the National Natural Science Foundation of China, No. 82160386 (to XY); General Project of Guangxi Natural Science Foundation of China, No. 2021GXNSFAA075042 (to XY)
摘要:
文题释义:
类装配体:是由多种类型细胞组成、具有空间结构的类器官,亦称为类组装体,它可比传统类器官更好地复制体内微环境、保持细胞的遗传物质和组织特点。自体组装:是指由干细胞增殖聚集,并被诱导分化形成含有多种细胞的类装配体的过程。
直接组装:指在体外直接将多种分化成熟的细胞按一定比例共培养,组装为类装配体的过程。
混合组装:指由干细胞形成的类器官或细胞球与分化成熟的不同细胞共培养,形成类装配体的过程。
背景:近年来,许多研究证实类装配体可弥补类器官无法完全重现细胞与细胞、细胞与基质间的互作关系的缺点,但处于发展初期的类装配体构建方式种类繁多,更无统一标准。
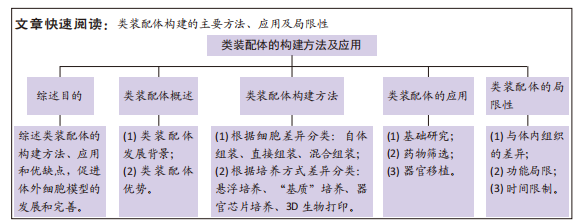
目的:综述目前类装配体的构建方法、应用和优缺点,为促进体外细胞模型的发展和完善提供指导。方法:以“assembloids,organoids,tumor microenvironment,organoids AND assemble,organoids AND microenvironment”为英文检索词,以“类装配体、类器官、类组装体、肿瘤微环境、类器官重组、多细胞模型”为中文检索词,检索PubMed、中国知网及万方数据库,在排除无关文章及去重后筛选出94篇文章进行综述。
结果与结论:①根据细胞来源的不同,可将类装配体的构建方法分为自体组装、直接组装及混合组装3种;根据细胞培养方式的差异,又可分为悬浮培养法、“基质”培养法、器官芯片培养法和3D生物打印法。②自体组装过程涵盖细胞和组织的发育等早期过程,因此,在器官发育和发育障碍等领域有广阔的前景,而分化成熟细胞的功能相对较完善,由它们直接组装成的类装配体在功能障碍及细胞损伤性疾病的研究中更具潜力;自体组装或在器官移植方面更胜一筹,直接组装将更适用于组织损伤的修复,混合组装综合了前两者的优势,多用于探索微环境中细胞的生理和病理机制以及药物筛选等领域。③虽然不同的类装配体各具优势,但都面临脉管系统不完善的难题;每种类装配体构建方法也存在各自的局限性,如自体组装形成的类装配体中细胞分化程度与体内的差异,直接组装模型的细胞种类固定、无法完全反映复杂的体内微环境等均是亟待解决的难题。④将来随着类装配体培养技术的不断完善,研究者们可以在体外组装出具有更复杂组织结构的仿生类器官,为研究人类组织和器官生理及病理过程提供无限趋近真实的模型。
https://orcid.org/0000-0002-4587-9640 (刘春磊);https://orcid.org/0000-0003-0226-9962 (姚茜) ;
https://orcid.org/0000-0002-8856-4243 (谢莹)
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
中图分类号:
引用本文
刘春磊, 姚 茜, 韦正波, 谢 莹. 类装配体的构建方法及应用[J]. 中国组织工程研究, 2024, 28(1): 113-120.
Liu Chunlei, , , Yao Xi, Wei Zhengbo, Xie Ying. Construction methods and application of assembloids[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(1): 113-120.
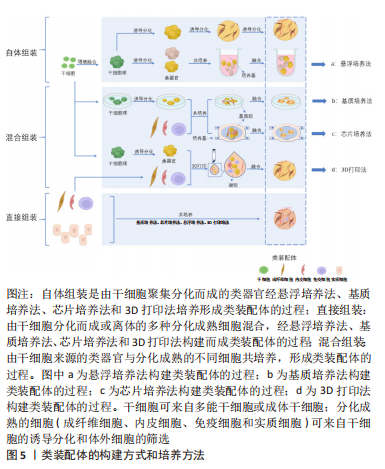
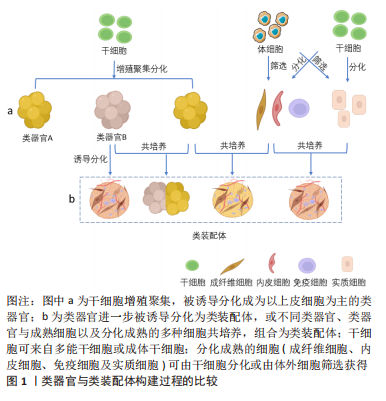
根据类装配体的发展历程,文章依据起始细胞的差异可将其构建方法分为:自体组装(self-assembly)、直接组装(direct- assembly)和混合组装(mixed-assembly),而每种构建方法又可因细胞培养方式的不同被分为:悬浮培养法、“基质”培养法、器官芯片培养法和3D生物打印法。
2.1.1 根据起始细胞分类
自体组装:是指由多能分化作用的干细胞增殖聚集,最终融合形成含有多种细胞的类装配体的过程。具体过程为:在培养基中添加促干细胞增殖以及刺激内皮细胞和免疫细胞等基质细胞产生的细胞因子,诱导干细胞分化出多种细胞的聚集体[25-26],或将干细胞增殖聚集并被诱导分化形成的不同球体融合,成为含有多种细胞的复合体[27-28],见图5。例如:选择感染有异位表达人类ETS变体2 (hEVT2)慢病毒的干细胞,并在其培养体系中加入神经分化因子,诱导出含有血管网络的人脑皮质类装配体[25],以及由包括脑源性神经营养因子(brain derived neurotrophic factor,BDNF)和神经营养因子3 (neurotrophin 3,NT3)在内的神经诱导因子刺激干细胞来源的大脑皮质获得脑皮质类器官,Smad抑制剂、视黄酸和音猬因子(sonic hedgehog,Shh)共同诱导分化出脊髓运动神经元类器官,并在含有BDNF和NT3等因子的培养基中诱导二者融合,最终再与预先获得的骨骼肌细胞在体外组装,形成运动系统[28]。
自体组装的优点:①干细胞具有多向分化潜能,对于模拟器官的生理发育机制更有优势;②构建过程较为简单,时间及物力的消耗较少。
自体组装的缺点:①脉管系统不完善[29],不利于模型中某些细胞的存活,对于在体外构建具有微环境的细胞模型来说,这是最为严峻的挑战;②存在细胞分化过程中异常通路被激活的可能性,这或许会导致细胞表型甚至组织异质性与体内的差异[30];③细胞的分化成熟程度与体内不一致[31-32],会导致功能欠缺,对于某些要求细胞分化程度较高且功能更完善的研究来说并不适用。由于自体组装的类装配体由干细胞起源,构建过程包括细胞的发育全程,因此其在细胞、组织和胚胎的发育研究、疾病发生和进展机制等领域具有很大优势[27-28,33-34]。
直接组装:直接组装则是根据实验需要利用多种分化成熟的细胞组装成为类装配体的过程。其构建经过如下:将预先获取的多种成熟细胞以合适的比例和方法共培养,并诱导它们融合成为多细胞复合体,见图5。这些分化成熟的细胞可来自干细胞的分化筛选或原代体细胞的筛选[35]。如MILLS教授[36]利用干细胞在含有骨形态发生蛋白4(bone
morphogenetic protein,BMP4)、激活素(activin A)和血管内皮生长因子 A (vascuoar endothelial growth factor A,VEGFA)等因子的培养基中分别进行分化,并以流式技术筛选出心肌细胞和内皮细胞,将二者以4∶1的比例共培养,组装成心脏类装配体,进一步用于验证溴结构域和末端外家族抑制剂(BETi)减少心肌细胞感染严重急性呼吸系统综合征冠状病毒2型(SARS-CoV-2)的机制。优点:由于直接组装的类装配体内含有多种分化成熟细胞,细胞的功能相对完善,因此使该模型具有更高的仿真度。缺点:除了具有脉管系统与体内不一致的局限性之外,直接组装的类装配体还存在细胞种类较固定、无法完整模拟体内组织的复杂性和异质性[35],以及构建过程较繁琐、周期相对较长的缺点。优势应用:在器官及细胞的功能性研究,如心功能异常的细胞机制探索和功能完善细胞模型探索的研究中有很大优势[20,36-38]。
混合组装:是指由干细胞增殖和聚集,并被诱导分化形成类器官或细胞球,再将这些类器官与分化成熟的不同细胞共培养,形成类装配体的过程[13,39],这里的干细胞包括多能干细胞、成体干细胞和肿瘤干细胞。如在含有人白细胞介素2和ROCK抑制剂Y-27632的培养基中诱导肠类器官与T细胞的共存,重构了免疫微环境[13]。近年来,这种方式多被用于重建肿瘤微环境,进一步探究肿瘤微环境中的免疫杀伤机制[40],以期开发更有效的免疫疗法。混合组装融合了上述两种方式的优点,如类装配体中的实质细胞由干细胞分化而来,而共培养系统中的成熟间质细胞又具有体内间质细胞的部分功能,这对于模拟组织、器官或肿瘤的发生发展非常有利。混合组装缺点也显而易见,脉管系统的不完善仍是最主要的,其次是细胞分化过程中异常通路被激活的可能性以及成熟间质细胞的种类较固定,使获得的类装配体与体内组织的差异有所减小,但仍不可忽视。优势应用:基质成分在组织发育中的作用机制[41]、肿瘤的发生发展和免疫微环境相关研究和药物筛选等领域[42-43]。
综上,上述3种类装配体构建方式之间最显著的区别在于培养过程中起始细胞的差异,其中,自体组装和混合组装过程含有干细胞,其具有无限增殖及多向分化潜能,所获得模型内的细胞将是较幼稚的;而直接组装所选用的分化成熟细胞具有功能完善的细胞器、信号通路以及与其他细胞或基质联系的受体和配体,因此,相对于自体和混合组装而言,直接组装模型功能更加完善,但由于已是分化成熟的细胞,丧失了分化潜能,导致其细胞种类较固定。除此之外,它们也存在其他缺点,见表1。掌握各种方式在不同研究中的优劣势,进一步完善类装配体的构建方法,提高其仿真程度,将为临床诊疗提供更有价值的实验结果。

2.1.2 根据培养方法分类
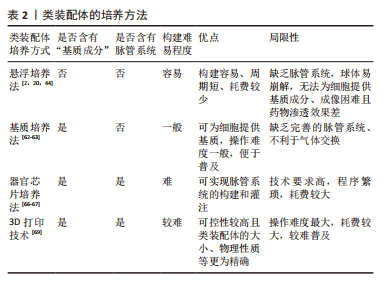
悬浮培养法:是最早被开发应用的方法,大致培养过程为:使用低黏附装置,将干细胞来源的不同细胞球或分化成熟的多种细胞在培养液中悬浮培养,添加特异的细胞因子,诱导它们融合为复合球体,见图5a。目前,此方法多以自体组装和定向组装进行类装配体的构建,如前所述,以干细胞分别诱导为脑皮质和脊髓类器官,随后在含有神经营养因子3和脑源性神经生长因子等因子的培养基中融合,最终与骨骼肌细胞共同形成运动环路[28]。在2012年,NAKANO团队[8]便运用此方法将干细胞培养出具有视锥细胞和视杆细胞等多种细胞的视杯模型。但也有研究发现使用该方法培养的细胞球体易崩解[44],构建效率较低。因此,为了提高悬浮培养的细胞成球率,许多学者尝试借助轨道振动器、超低附着板或旋转生物反应器等设备[25,45-46],以干细胞自体组装培养出大脑[12,14]、心脏等器官和肿瘤的类装配体[17,47-49]。上述均为以干细胞进行的自体组装,除此之外,也有学者利用分化成熟的细胞共培养而直接组装成类装配体[50-51]。该方法操作简单、周期较短,因此是最早尝试的3D培养法,使细胞培养具备三维空间结构成为可能,在探究神经系统疾病和心脏发育方面做出了重大贡献[12,47],并为后续类装配体培养方式的发展奠定了基础。虽然这种方法培养的类装配体无法为细胞提供基质成分,但其构建细胞球体过程简单且耗时短,将其培养出的细胞球体与其他方法结合,是一种更高效的类装配体构建方法,而成像困难且药物渗透效果差等缺点[2],使其在基础研究中并未被广泛应用。
“基质”培养法:是对悬浮培养法的改良,以生物材料作为“基质”,为细胞提供支持,将不同类器官及多种细胞两两植入其中[13,39,52],组合成为类装配体,见图5b。此方法弥补了悬浮培养法成球率低、渗透性差、易破碎等缺陷,还为细胞的生长提供了“基质”,在重建肿瘤微环境、构建发育更成熟的“器官”领域广受欢迎。其中,基质胶是应用最为广泛的生物材料,目前多以此方法进行混合组装和直接组装构建类装配体,自体组装相对较少。例如,一些学者在基质胶中将干细胞聚集分化形成的类器官与成熟的基质细胞共培养,成功构建出肠道[53]、肿瘤等混合组装的类装配体[40],以及多种成熟细胞相互融合而直接组装的心脏[36,54]、肝脏[18,38]、胰腺[20,55]、脉管和肾脏等类装配体[18,56]。但是,细胞的生长和增殖除了需要营养物质外,也需要充足的氧气,许多研究发现单纯在基质胶中培养无法维持免疫细胞等多种细胞的长时间存活[4,26],这或许是由于基质胶等生物材料完全浸没于培养液中不利于气体交换的原因。在2011年,PRYTHERCH教授[57]以气液界面法培养出人支气管上皮模型,尝试解决基质胶培养法中缺乏气体交换功能的问题,发现在气液界面中可分化出更多种类的支气管细胞。该方法与单纯基质胶培养的不同之处在于含有类装配体的基质胶不完全浸没于培养液中,形成气液平面,使该模型实现了部分气体交换功能[58],从而使细胞能更好的保持自身表型[59]。NEAL教授[4]在其研究中证实了这一成果,其研究结果表明肿瘤微环境中的免疫细胞在类装配体中可存活长达近2个月,且保留了自身原始的T细胞受体谱。另外,在类装配体中形成具有器官特异性的结构方面,此方法也相对于基质胶法更优,如GUPTA教授[60]发现以该方法组装的肾脏类器官具有脉管内皮及肾小管结构,这将在疾病模型构建及药物筛选方面发挥更大的作用。
虽然基质胶培养法在类装配体构建方法的发展进程中具有重要的阶梯作用,但是商业化的生物材料如基质胶来源于小鼠,其本质为小鼠肉瘤的基底膜提取物,富含细胞外基质蛋白,因此有许多研究发现,在构建含有免疫细胞,尤其是CD4+T细胞的类装配体过程中易触发免疫细胞发生交叉免疫反应[40]。相对于传统的商业化生物材料而言,去细胞基质具有更好的组织相容性。因此,一些学者尝试利用去细胞化的基质为支架,将干细胞或类器官和基质细胞注入其中进行培养[21,61],以自体或混合组装方式构建类装配体,如将脂肪干细胞注入无细胞的猪膀胱基质中获得可排尿的膀胱模型[21],MERAN团队[61]则将肠道类器官和成纤维细胞一起注入无细胞肠道基质中,最终培养出具有生理功能的小肠类装配体。去细胞基质可选择自体或同种异体来源的组织制成,进一步提高组织相容性,这对于缓解器官移植供源不足以及建立同种异体移植物的免疫耐受性具有重要的指导意义。
目前,“基质”培养法是最受欢迎的方式,可为细胞和类装配体提供适宜的生长环境,如细胞外“基质”和相对充足的氧气。但因其缺乏体内组织中均匀分布的脉管网络以及灌注功能[62-63],导致细胞模型仍不能完全模拟人体组织的整体功能,这是目前装配体发展所面临的最大难题。
器官芯片培养法:众所周知,人体微环境与疾病进程息息相关,而脉管系统是体内微环境不可缺少的一部分,也是类装配体构建过程中的重点和难点,可能是因为其中缺少内皮细胞成管所需的附着物、流动的基质成分及基质细胞等[29],而导致无法形成“微脉管”。已有许多研究证实缺乏脉管系统会导致体积较大类装配体内部细胞缺氧坏死[62,64]。器官芯片主要用于在模型中实现流动的“基质”,为脉管系统的构建提供可能,甚至模拟体内的物理化学微环境和血液循环,此外,它还具有标准且大小可控的腔室,可模拟体内的多细胞层结构并进行高通量扩增[65]。该方法是将器官芯片与立体细胞模型培养技术相结合,使类器官与基质细胞在器官芯片中共培养,见图5c,通过器官芯片外循环系统提供动力促进培养液循环流动,可使内皮细胞在连续灌注条件下形成具有管状结构的“脉管”。已有大量文献表明,器官芯片可为脉管系统的重建提供条件,促进微脉管网络形成[66],如以脑类器官与内皮细胞共培养构建血管化的混合组装类装配体[67],并评估了血管的灌注和通透性[41],这可能与内皮细胞和其他基质细胞中存在固定的生物程序,指导内皮细胞自组装形成脉管网络有关[29],将促进细胞间、细胞与流动基质间的相互作用。
类装配体芯片是医学生物学与工程学结合的产物,充分融合了两者的优势,有效解决了上述类装配体培养法存在的诸多局限性。相比其他构建方法,器官芯片可为类装配体提供“流动的基质”,以及标准化的物理性质,在类装配体的探索进程中具有里程碑式的意义。至此,类装配体的结构和功能与人体组织更相仿,在细胞及器官发育、疾病发生发展、药物筛选以及再生等领域具有无可比拟的优势。然而,由于该方法培养平台制作程序繁琐且困难,造价较高,终使其难以推广。
3D生物打印:3D生物打印作为新兴的仿生技术,正在逐渐与3D细胞培养方法结合。相对于上述方法来说,在培养多种细胞融合为类装配体时,3D生物打印具有更大的优势,不仅实现了从手动到自动的转变,而且在保证细胞模型仿生程度的同时又可实现精准控制,便于进行高通量培养,这些优势将推动细胞模型发生突破性进展。
目前,3D生物打印技术主要是以生物材料与类器官或不同的细胞为原料进行自动组装构建类装配体[68],通过设置可行的参数,保证打印的可重复性、精确性和可靠性[69],从而实现类装配体与人体组织的高度一致,见图5d。传统细胞模型以及类器官在构建过程中并不能很好地控制细胞的密度、模型大小以保证良好的重复性,这对于严谨的生命科学实验来说,并不乐观。而3D生物打印却能很好的解决这些难题,如LAWLOR团队[69]的研究表明以类器官进行3D生物打印可增加构建过程的可控性,最终自体组装形成成熟度更高的肾类装配体。HUMPHREYS教授[70]进一步以3D生物打印技术构建出高稳定性和可重复性的肾类装配体。除此之外,3D生物打印在细胞模型微环境和结构的重建中也颇显优势,如BRASSARD等[71]以肠类器官打印出含有肠腔、分支脉管、小肠隐窝和绒毛结构等的高度仿生厘米级的肠类装配体,可精确构建出与体内组织生理仿真性更相似的空间结构[69]。而通过直接组装打印出的血管化心脏类装配体具有更高的组织相容性[72],并具备收缩功能。在混合组装中,3D生物打印出的类装配体可提高细胞的活力[73],在探究肿瘤细胞可塑性的新机制方面表现出巨大潜力。未来,在模拟体内组织的生理特点及功能方面,此方法将比其他方法更胜一筹,打印出细胞分布、组成和结构与真实器官一致的“微器官”,或可进行多器官多系统类装配体的同步构建,为基础研究和临床研究提供更真实的平台。3D打印技术优点突出且众多,但其依靠先进的科学技术[72-73],因此将耗费巨大的财力,这是其不能普及的主要原因。
文章总结了类装配体的培养方法优缺点,见表2。
细胞差异是构建方式差异的本质,其成熟程度和种类将会影响实质细胞的表型和功能,使实验研究结果产生偏差,在基础研究中不容忽视。在细胞差异的基础上,细胞培养方法的不同将进一步影响实验结果。目前来说,上述4种培养方法为主的细胞培养策略各具优势,而针对各种器官模型的培养方法尚在探索之中,将来的不同类装配体或将具有各自统一的器官特异性培养标准。无论以何种方式构建的类装配体,总体来说都具有既往模型无法比拟的优点,是细胞模型发展的重要进步。在充分评估各种方式优缺点的同时,针对不同的科学研究选择合适的方法,将挖掘出该模型的最大潜力。
2.2 类装配体的应用
2.2.1 基础研究 类装配体是对类器官的完善和发展,弥补了传统类器官细胞类型单一、缺乏微环境的缺点,其除了被广泛应用于生理发育机制研究和疾病造模等基础研究之外[27,33-34,74],还凭借其在重建细胞间联系及微环境方面超越传统类器官的优势[4,28,52],从全局出发研究体内的生理或病理机制[36,75],进而提高临床转化应用率。但以3种方式构建的类装配体所选用的起始细胞存在差异,因而应用领域各有侧重。自体组装以干细胞为起点,更适用于监测细胞发育全程、模拟器官早期发育等研究。而直接组装以分化成熟的细胞为起点,可获得功能更为完善的细胞模型,更多被应用于功能探索性研究,如心脏模型构建和心肌细胞功能检测等[37]。混合组装综合了二者的优势,即可被用于探究细胞、组织的发育机制[41],也可在疾病的病理过程等研究中提供准确的指导[40],使细胞模型的生长过程更接近人体内组织,或许会以此获得更准确的实验结果。总之,以类装配体为平台进行科学研究,更利于从全局出发探索体内的生理病理机制,在疾病的发生发展及转归等相关研究中具有重大意义。随着构建方法的发展,细胞模型中的脉管系统和细胞种类也将更加完善,进一步促进基础研究的发展。
2.2.2 药物筛选 药敏模型是连接基础研究与临床的桥梁,对于提高药敏试验和新药研发的可信度至关重要。已有大量研究表明肿瘤治疗效果与肿瘤微环境密切相关[76],其中包含大量肿瘤微环境相关的治疗靶点,但却在临床转化应用中进展缓慢,其根本原因在于现有的细胞模型,尚无法重现与体内组织完全一致的肿瘤微环境,不能成为患者的精准“替身”。肿瘤类装配体除了含有肿瘤细胞,还包含维持肿瘤细胞增殖、浸润、迁移能力甚至耐药性的间质细胞,如成纤维细胞和免疫细胞等,相对于只含有单一上皮肿瘤细胞的模型,类装配体在体外为患者“试药”更具优势[4,42]。而在非肿瘤性组织中,目前用于药物实验的模型以直接组装的类装配体较为可靠,如心脏疾病的药物疗效和血脑屏障类器官对药物的筛选等[36-37,51],主要原因是这些直接组装模型具有分化程度更高功能更完善的细胞,对药物的反应更接近于体内效应。因此,根据实验的需要选择不同的方法构建类装配体,对于药物的筛选具有很大帮助。
2.2.3 器官移植 器官移植可改善终末期患者的生存质量,除极其严格的筛选程序外,供体来源不足一直是限制其应用的原因。在该领域,类器官一直被寄予厚望,但目前其培养技术仍未完全成熟。相对于类器官,自体组装类装配体的细胞种类和组织异质性方面与体内器官更相似,在器官再生领域更具有前景[21,77],并且以无细胞基质为支架和3D生物打印构建的类装配体具有更好的免疫耐受性[78]。而对于心脏、骨骼肌等含有稳定实质细胞的器官来说,直接组装的“器官”具有更完整的功能[79],或许在组织修复领域更加适用;同样,对于功能完善且细胞较活跃的器官来说,以混合组装构建类装配体进行损伤的修复将不失为一种好方法。在将来,类装配体将具有器官特异性的微环境,在模拟器官的生理机制方面还原程度更高,将为器官移植和组织修复提供功能更完善的“器官”和“组织”。见表3。
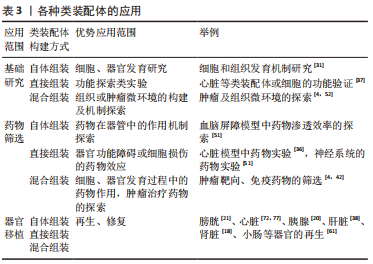
总的来说,类装配体在重建微环境和细胞间、细胞与基质间联系方面有较大潜力,但由于不同构建方法细胞分化和培养方式的差异,导致各种类装配体具有各自的优缺点,最终使它们适宜的应用领域也有所不同。因此,根据不同的研究目的选择适宜的细胞来源及培养方法,将更有利于研究的开展。未来,类装配体将成为无限趋近于真实器官和组织的体外模型应用于生理、病理机制研究,成为患者的“替身”开展精准的个体医疗,成为自体器官移植的来源,解决目前供体不足的困境。
2.3 类装配体的局限性 类装配体发展至今,显著解决了现有体外模型细胞类型单一、缺乏微环境的问题,实现了细胞间、细胞与基质间联系的信号传递,是目前最为理想的细胞模型。但与人体器官相比,在组织异质性、功能和时效性方面仍存在一定差异[80],这可能影响部分实验的结果。基于现有的构建方法,分析总结类装配体存在的缺点,对其未来的发展意义重大。
2.3.1 类装配体与体内组织的差异 与体内组织的差异在细胞模型中最为常见,因构建方式的不同而各异,但总的来说,类装配体和体内组织之间主要是脉管系统和干细胞分化过程的差异。目前,体外模型的“脉管”与体内脉管之间既存在物理性质,如截面和分布的差异[43,81-82],又存在气体交换等功能的差异[83]。这会导致模型中的液体流动方式以及“血供”比例与体内不符,使“血供”较少部位的细胞缺氧坏死[62,64],以及基质细胞如免疫细胞的数量减少[26]。虽然这可用来构建肿瘤缺氧模型[84],但却不利于细胞的生长,旋转生物反应器的使用对球体内部缺氧的改善也并不明显[85]。随着技术发展,已有文献报道将3D生物打印技术[70]、器官芯片技术和类装配体培养技术融合[29,41],可成功构建出具有脉管系统的类装配体,且部分研究发现这些脉管具有灌注功能[29]。
除了脉管系统差异外,在构建类装配体的过程中,尤其在自体组装过程中,干细胞增殖分化的同时也伴随着某些信号途径被异常激活,最终导致分化或表型差异[30],这将严重影响体外实验结果的可靠性。针对此类问题,将信号传导中心引入类装配体构建的全程[86-88],如Shh蛋白梯度系统在器官发生中起着关键作用,使人脑立体细胞模型能够有序自组织,形成细化的前脑组织,并且与内脑组织行为一致[86]。信号调控中心可指导细胞分化、组装和功能完善,是未来的研究方向。组织差异是细胞模型无限接近体内组织进程中务须解决的难题,其中脉管的构建尤其重要,目前来说,以器官芯片构建的细胞模型可形成较接近体内脉管网络的“微脉管”,因此,以器官芯片为主联合气液界面或无细胞基质的方法将培养出更逼真的模型,并构建出器官特异性的脉管系统,这对于基础研究甚至临床前研究尤其重要。
2.3.2 类装配体的功能局限性 器官的功能需要功能完善的细胞聚集形成空间结构来实现,然而,虽然类装配体具有空间结构,但并没有与体内器官成熟一致的细胞[53],这也会对研究结果产生影响。例如,由干细胞来源的心肌细胞与胎儿心肌细胞的形态、表型和电生理活动相似[89],导致类装配体中的心肌细胞不足以行使成熟心肌细胞的功能。因此,除了要不断完善微环境之外,也需要进一步探索促进和维持细胞分化状态的培养方式。在探索血管化细胞模型的过程中,已有学者发现体内基质成分尤其是脉管的存在会促进类装配体的成熟,并增强其功能[25,32,41]。然而,在类装配体中构建脉管系统始终是个难题,尤其是构建具有功能的脉管系统。另外,一些学者尝试在体外以电刺激和机械刺激促进心肌细胞的成熟[79,90],并有所成效。类似地,也可尝试探索以器官特异性的刺激促进各种类装配体的成熟,如与原尿渗透压一致的培养液促进肾小球模型的成熟、与血糖浓度相仿的培养基糖浓度诱导胰腺模型的成熟等,加之脉管系统的完善,将促进类装配体的细胞存活时间延长、功能更加完善,显著提高实验结果的临床转化率。
2.3.3 类装配体的时效稳定性 虽然类装配体含有多种细胞,可为细胞的存活和表型验证提供良好的平台,但在目前类装配体的构建过程中,存在某些细胞的存活时间较短,或类装配体构建周期较长的缺陷,如免疫细胞在体系中最长的存活时间为60 d[4],这远不能满足将来学者们以类装配体进行“多系统或多器官”的整体性研究。因此,进一步加快类装配体的组装、缩短周期、延长各种细胞的存活时间将其在基础研究甚至临床研究中大放异彩[91]。
综上,无论以何种方法构建的类装配体,均与体内组织存在一定差异,其中细胞种类及成熟程度、基质成分的不完善以及培养方法的不同是导致这些差异的重要原因,此外还存在细胞及基质运动方式以及细胞所处物理环境等因素的影响。多种构建方式或培养方法的联合,可能是减少其与人体组织差异的有效手段,随着类器官培养技术的不断完善,现有的局限性被逐一克服,将来多系统多器官细胞模型的出现将进一步推动基础实验及临床研究应用平台向整体化、系统化方向发展,必将在精准医疗发展中发挥更大作用。

| [1] SATO T, VRIES RG, SNIPPERT HJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459(7244): 262-265. [2] FITZGERALD AA, LI E, WEINER LM. 3D Culture systems for exploring cancer immunology. Cancers. 2020;13(1):56. [3] VLACHOGIANNIS G, HEDAYAT S, VATSIOU A, et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science. 2018;359(6378):920-926. [4] NEAL JT, LI X, ZHU J, et al. Organoid modeling of the tumor immune microenvironment. Cell. 2018;175(7):1972-1988.e16. [5] KANTON S, PAŞCA SP. Human assembloids. Development. 2022;149(20): dev201120. [6] YIN X, MEAD BE, SAFAEE H, et al. Engineering stem cell organoids. Cell Stem Cell. 2016;18(1):25-38. [7] TAKUMA H, SAKURAI M, KANAZAWA I. In vitro formation of corticospinal synapses in an organotypic slice co-culture. Neuroscience. 2002;109(2): 359-370. [8] NAKANO T, ANDO S, TAKATA N, et al. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell. 2012; 10(6):771-785. [9] BIREY F, ANDERSEN J, MAKINSON CD, et al. Assembly of functionally integrated human forebrain spheroids. Nature. 2017;545(7652):54-59. [10] 周珍珍,庞媛,孙伟.肿瘤类装配体“镜像”重现肿瘤微环境[J].科学通报,2021,66(34):4348-4349. [11] KIM E, CHOI S, KANG B, et al. Creation of bladder assembloids mimicking tissue regeneration and cancer. Nature. 2020;588(7839):664-669. [12] MIURA Y, LI MY, REVAH O, et al. Engineering brain assembloids to interrogate human neural circuits. Nat Protoc. 2022;17(1):15-35. [13] TAKASHIMA S, MARTIN ML, JANSEN SA, et al. T cell-derived interferon-γ programs stem cell death in immune-mediated intestinal damage. Sci Immunol. 2019;4(42):eaay8556. [14] ORMEL PR, VIEIRA DE Sá R, VAN BODEGRAVEN EJ, et al. Microglia innately develop within cerebral organoids. Nat Commun. 2018;9(1):4167. [15] HOFBAUER P, JAHNEL SM, PAPAI N, et al. Cardioids reveal self-organizing principles of human cardiogenesis. Cell. 2021;184(12):3299-3317.e3222. [16] LEE J, SUTANI A, KANEKO R, et al. In vitro generation of functional murine heart organoids via FGF4 and extracellular matrix. Nat Commun. 2020; 11(1):4283. [17] DRAKHLIS L, BISWANATH S, FARR CM, et al. Human heart-forming organoids recapitulate early heart and foregut development. Nat Biotechnol. 2021; 39(6):737-746. [18] TAKEBE T, ENOMURA M, YOSHIZAWA E, et al. Vascularized and complex organ buds from diverse tissues via mesenchymal cell-driven condensation. Cell Stem Cell. 2015;16(5):556-565. [19] BELOW CR, KELLY J, BROWN A, et al. A microenvironment-inspired synthetic three-dimensional model for pancreatic ductal adenocarcinoma organoids. Nat Mater. 2022;21(1):110-119. [20] AUGSORNWORAWAT P, VELAZCO-CRUZ L, SONG J, et al. A hydrogel platform for in vitro three dimensional assembly of human stem cell-derived islet cells and endothelial cells. Acta Biomater. 2019;97;272-280. [21] POKRYWCZYNSKA M, JUNDZILL A, RASMUS M, et al. Understanding the role of mesenchymal stem cells in urinary bladder regeneration-a preclinical study on a porcine model. Stem Cell Res Ther. 2018;9(1):328. [22] KUMAR V, RAMNARAYANAN K, SUNDAR R, et al. Single-cell atlas of lineage states, tumor microenvironment, and subtype-specific expression programs in gastric cancer. Cancer Discov. 2022;12(3):670-691. [23] MAHATO S, AGRAWAL T, PIDISHETTY D, et al. Generation of retinal organoids from healthy and retinal disease-specific human-induced pluripotent stem cells. J Vis Exp. 2022. doi: 10.3791/64509. [24] ABRAHAM DM, HERMAN C, WITEK L, et al. Self-assembling human skeletal organoids for disease modeling and drug testing. J Biomed Mater Res B Appl Biomater. 2022;110(4):871-884. [25] CAKIR B, XIANG Y, TANAKA Y, et al. Engineering of human brain organoids with a functional vascular-like system. Nat Methods. 2019;16(11):1169-1175. [26] JACOB F, SALINAS RD, ZHANG DY, et al. A patient-derived glioblastoma organoid model and biobank recapitulates inter- and intra-tumoral heterogeneity. Cell. 2020;180(1):188-204.e122. [27] BIREY F, LI MY, GORDON A, et al. Dissecting the molecular basis of human interneuron migration in forebrain assembloids from Timothy syndrome. Cell Stem Cell. 2022;29(2):248-264.e247. [28] ANDERSEN J, REVAH O, MIURA Y, et al. Generation of functional human 3D cortico-motor assembloids. Cell. 2020;183(7):1913-1929.e26. [29] SHIRURE VS, HUGHES CCW, GEORGE SC. Engineering vascularized organoid-on-a-chip models. Annu Rev Biomed Eng. 2021;23:141-167. [30] BHADURI A, ANDREWS MG, MANCIA LEON W, et al. Cell stress in cortical organoids impairs molecular subtype specification. Nature. 2020;578(7793): 142-148. [31] SLOAN SA, ANDERSEN J, PAȘCA AM, et al. Generation and assembly of human brain region–specific three-dimensional cultures. Nature Protocols. 2018;13(9):2062-2085. [32] ZAHMATKESH E, KHOSHDEL-RAD N, MIRZAEI H, et al. Evolution of organoid technology: lessons learnt in Co-Culture systems from developmental biology. Dev Biol. 2021;475:37-53. [33] FAUSTINO MARTINS JM, FISCHER C, URZI A, et al. Self-organizing 3D human trunk neuromuscular organoids. Cell Stem Cell. 2020;26(2):172-186.e176. [34] VICTOR MB, LEARY N, LUNA X, et al. Lipid accumulation induced by APOE4 impairs microglial surveillance of neuronal-network activity. Cell Stem Cell. 2022;29(8):1197-1212.e1198. [35] LEWIS-ISRAELI YR, WASSERMAN AH, AGUIRRE A. Heart organoids and engineered heart tissues: novel tools for modeling human cardiac biology and disease. Biomolecules. 2021. doi: 10.3390/biom11091277. [36] MILLS RJ, HUMPHREY SJ, FORTUNA PRJ, et al. BET inhibition blocks inflammation-induced cardiac dysfunction and SARS-CoV-2 infection. Cell. 2021;184(8):2167-2182.e2122. [37] ZHAO Y, RAFATIAN N, FERIC NT, et al. A platform for generation of chamber-specific cardiac tissues and disease modeling. Cell. 2019; 176(4):913-927.e918. [38] GOULART E, DE CAIRES-JUNIOR LC, TELLES-SILVA KA, et al. Adult and iPS-derived non-parenchymal cells regulate liver organoid development through differential modulation of Wnt and TGF-β. Stem Cell Res Ther. 2019;10(1):258. [39] RAWLINGS TM, MAKWANA K, TAYLOR DM, et al. Modelling the impact of decidual senescence on embryo implantation in human endometrial assembloids. Elife. 2021. doi: 10.7554/eLife.69603. [40] DIJKSTRA KK, CATTANEO CM, WEEBER F, et al. Generation of tumor-reactive t cells by co-culture of peripheral blood lymphocytes and tumor organoids. Cell. 2018;174(6):1586-1598.e1512. [41] SALMON I, GREBENYUK S, ABDEL FATTAH AR, et al. Engineering neurovascular organoids with 3D printed microfluidic chips. Lab Chip. 2022; 22(8):1615-1629. [42] VOTANOPOULOS KI, FORSYTHE S, SIVAKUMAR H, et al. Model of patient-specific immune-enhanced organoids for immunotherapy screening: feasibility study. Ann Surg Oncol. 2020;27(6):1956-1967. [43] MARTON RM, PAȘCA SP. Organoid and assembloid technologies for investigating cellular crosstalk in human brain development and disease. Trends Cell Biol. 2020;30(2):133-143. [44] BYRNE AT, ALFéREZ DG, AMANT F, et al. Interrogating open issues in cancer medicine with patient-derived xenografts. Nat Rev Cancer. 2017;17(10):632. [45] SONG L, YUAN X, JONES Z, et al. Assembly of human stem cell-derived cortical spheroids and vascular spheroids to model 3-D brain-like tissues. Sci Rep. 2019;9(1):5977. [46] KRENCIK R, SEO K, VAN ASPEREN JV, et al. Systematic three-dimensional coculture rapidly recapitulates interactions between human neurons and astrocytes. Stem Cell Reports. 2017;9(6):1745-1753. [47] ROSSI G, BROGUIERE N, MIYAMOTO M, et al. Capturing cardiogenesis in gastruloids. Cell Stem Cell. 2021;28(2):230-240.e236. [48] LEWIS-ISRAELI YR, WASSERMAN AH, GABALSKI MA, et al. Self-assembling human heart organoids for the modeling of cardiac development and congenital heart disease. Nat Commun. 2021;12(1):5142. [49] LINKOUS A, BALAMATSIAS D, SNUDERL M, et al. Modeling patient-derived glioblastoma with cerebral organoids. Cell Rep. 2019;26(12):3203-3211.e05. [50] RICHARDS DJ, LI Y, KERR CM, et al. Human cardiac organoids for the modelling of myocardial infarction and drug cardiotoxicity. Nat Biomed Eng. 2020;4(4):446-462. [51] BERGMANN S, LAWLER SE, QU Y, et al. Blood–brain-barrier organoids for investigating the permeability of CNS therapeutics. Nat Protoc. 2018; 13(12):2827-2843. [52] BIFFI G, ONI TE, SPIELMAN B, et al. IL1-induced JAK/STAT signaling is antagonized by TGFβ to shape CAF heterogeneity in pancreatic ductal adenocarcinoma. Cancer Discov. 2019;9(2):282-301. [53] JUNG KB, LEE H, SON YS, et al. Interleukin-2 induces the in vitro maturation of human pluripotent stem cell-derived intestinal organoids. Nat Commun. 2018;9(1):3039. [54] VARZIDEH F, PAHLAVAN S, ANSARI H, et al. Human cardiomyocytes undergo enhanced maturation in embryonic stem cell-derived organoid transplants. Biomaterials. 2019;192:537-550. [55] CANDIELLO J, GRANDHI TSP, GOH SK, et al. 3D heterogeneous islet organoid generation from human embryonic stem cells using a novel engineered hydrogel platform. Biomaterials. 2018;177:27-39. [56] WIMMER RA, LEOPOLDI A, AICHINGER M, et al. Human blood vessel organoids as a model of diabetic vasculopathy. Nature. 2019;565(7740): 505-510. [57] PRYTHERCH Z, JOB C, MARSHALL H, et al. Tissue-Specific stem cell differentiation in an in vitro airway model. Macromol Biosci. 2011;11(11): 1467-1477. [58] LI X, OOTANI A, KUO C. An air-liquid interface culture system for 3d organoid culture of diverse primary gastrointestinal tissues. Methods Mol Biol. 2016; 1422:33-40. [59] GIANDOMENICO SL, MIERAU SB, GIBBONS GM, et al. Cerebral organoids at the air-liquid interface generate diverse nerve tracts with functional output. Nat Neurosci. 2019;22(4):669-679. [60] GUPTA AK, IVANCIC DZ, NAVED BA, et al. An efficient method to generate kidney organoids at the air-liquid interface. J Biol Methods. 2021;8(2):e150. [61] MERAN L, MASSIE I, CAMPINOTI S, et al. Engineering transplantable jejunal mucosal grafts using patient-derived organoids from children with intestinal failure. Nat Med. 2020;26(10):1593-1601. [62] GRONHOLM M, FEODOROFF M, ANTIGNANI G, et al. Patient-derived organoids for precision cancer immunotherapy. Cancer Res. 2021;81(12): 3149-3155. [63] GREBENYUK S, RANGA A. Engineering organoid vascularization. Front Bioeng Biotechnol. 2019;7:39. [64] ZHANG S, WAN Z, KAMM RD. Vascularized organoids on a chip: strategies for engineering organoids with functional vasculature. Lab Chip. 2021;21(3): 473-488. [65] TSAI HF, TRUBELJA A, SHEN AQ, et al. Tumour-on-a-chip: microfluidic models of tumour morphology, growth and microenvironment. J R Soc Interface. 2017;14(131):1277. [66] JONES CFE, DI CIO S, CONNELLY JT, et al. Design of an integrated microvascularized human skin-on-a-chip tissue equivalent model. Front Bioeng Biotechnol. 2022;10:915702. [67] SHIN N, KIM Y, KO J, et al. Vascularization of iNSC spheroid in a 3D spheroid-on-a-chip platform enhances neural maturation. Biotechnol Bioeng. 2022; 119(2):566-574. [68] BANERJEE D, SINGH YP, DATTA P, et al. Strategies for 3D bioprinting of spheroids: a comprehensive review. Biomaterials. 2022;291;121881. [69] LAWLOR KT, VANSLAMBROUCK JM, HIGGINS JW, et al. Cellular extrusion bioprinting improves kidney organoid reproducibility and conformation. Nat Mater. 2021;20(2):260-271. [70] HUMPHREYS BD. Bioprinting better kidney organoids. Nat Mater. 2021; 20(2):128-130. [71] BRASSARD JA, NIKOLAEV M, HüBSCHER T, et al. Recapitulating macro-scale tissue self-organization through organoid bioprinting. Nat Mater. 2021; 20(1):22-29. [72] ZHANG YS, ARNERI A, BERSINI S, et al. Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials. 2016;110:45-59. [73] HEO DN, AYAN B, DEY M, et al. Aspiration-assisted bioprinting of co-cultured osteogenic spheroids for bone tissue engineering. Biofabrication. 2020;13(1). doi: 10.1088/1758-5090/abc1bf. [74] MARTON RM, MIURA Y, SLOAN SA, et al. Differentiation and maturation of oligodendrocytes in human three-dimensional neural cultures. Nat Neurosci. 2019;22(3):484-491. [75] EURA N, MATSUI TK, LUGINBüHL J, et al. Brainstem organoids from human pluripotent stem cells. Front Neurosci. 2020;14:538. [76] KLEMM F, JOYCE JA. Microenvironmental regulation of therapeutic response in cancer. Trends Cell Biol. 2015;25(4):198-213. [77] STOWER H. Bioprinting a human heart. Nature Medicine. 2019;25(9): 1330-1330. [78] FAN Y, TAJIMA A, GOH SK, et al. Bioengineering thymus organoids to restore thymic function and induce donor-specific immune tolerance to allografts. Mol Ther. 2015;23(7):1262-1277. [79] RONALDSON-BOUCHARD K, MA SP, YEAGER K, et al. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature. 2018; 556(7700):239-243. [80] KOO B, CHOI B, PARK H, et al. Past, present, and future of brain organoid technology. Mol Cells. 2019;42(9):617-627. [81] PHAN DT, BENDER RHF, ANDREJECSK JW, et al. Blood-brain barrier-on-a-chip: Microphysiological systems that capture the complexity of the blood-central nervous system interface. Exp Biol Med (Maywood). 2017;242(17):1669-1678. [82] XIA T, DU WL, CHEN XY, et al. Organoid models of the tumor microenvironment and their applications. J Cell Mol Med. 2021;25(13):5829-5841. [83] QIAN X, SU Y, ADAM CD, et al. Sliced human cortical organoids for modeling distinct cortical layer formation. Cell Stem Cell. 2020;26(5):766-781.e769. [84] BHATTACHARYA S, CALAR K, DE LA PUENTE P. Mimicking tumor hypoxia and tumor-immune interactions employing three-dimensional in vitro models. J Exp Clin Cancer Res. 2020;39(1):75. [85] QIAN X, NGUYEN HN, SONG MM, et al. Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell. 2016;165(5):1238-1254. [86] CEDERQUIST GY, ASCIOLLA JJ, TCHIEU J, et al. Specification of positional identity in forebrain organoids. Nat Biotechnol. 2019;37(4):436-444. [87] MIYAMOTO M, NAM L, KANNAN S, et al. Heart organoids and tissue models for modeling development and disease. Semin Cell Dev Biol. 2021;118:119-128. [88] KHOSHDEL RAD N, AGHDAMI N, MOGHADASALI R. Cellular and molecular mechanisms of kidney development: from the embryo to the kidney organoid. Front Cell Dev Biol. 2020;8:183. [89] FERIC NT, RADISIC M. Maturing human pluripotent stem cell-derived cardiomyocytes in human engineered cardiac tissues. Adv Drug Deliv Rev. 2016;96;110-134. [90] LU K, SEIDEL T, CAO-EHLKER X, et al. Progressive stretch enhances growth and maturation of 3D stem-cell-derived myocardium. Theranostics. 2021; 11(13):6138-6153. [91] SEKIYA S, KIKUCHI T, SHIMIZU T. Perfusion culture maintained with an air-liquid interface to stimulate epithelial cell organization in renal organoids in vitro. BMC Biomed Eng. 2019;1:15. [92] SHPICHKA A, BIKMULINA P, PESHKOVA M, et al. Engineering a model to study viral infections: bioprinting, microfluidics, and organoids to defeat coronavirus disease 2019 (COVID-19). Int J Bioprint. 2020;6(4):302. [93] KELLEY KW, PAȘCA SP. Human brain organogenesis: toward a cellular understanding of development and disease. Cell. 2022;185(1):42-61. [94] DROST J, CLEVERS H. Organoids in cancer research. Nat Rev Cancer. 2018; 18(7):407-418. |
| [1] | 孙可欣, 曾今实, 李佳, 蒋海越, 刘霞. 力学刺激提高生物3D打印软骨构建物基质的形成[J]. 中国组织工程研究, 2023, 27(在线): 1-7. |
| [2] | 宋美玲, 李征宇, 艾子政, 李京娜, 曾庆丰, 韩倩倩, 董谢平. 不同比例羟基磷灰石/β-磷酸三钙涂层支架修复骨缺损[J]. 中国组织工程研究, 2023, 27(30): 4809-4816. |
| [3] | 蒋鸿辉, 孔媛媛, 刘 婧, 王志红. 制备脱细胞基质生物墨水在心血管疾病领域中的应用[J]. 中国组织工程研究, 2023, 27(30): 4904-4911. |
| [4] | 孙可欣, 曾今实, 李 佳, 蒋海越, 刘 霞. 力学刺激提高生物3D打印软骨构建物基质的形成[J]. 中国组织工程研究, 2023, 27(21): 3293-3299. |
| [5] | 路冬冬, 朱天峰, 张一健, 赵志坚, 刘 洋, 沈 序, 朱雪松. 3D生物打印甲基丙烯酰化明胶水凝胶支架促进软骨下骨缺损的修复[J]. 中国组织工程研究, 2022, 26(34): 5454-5460. |
| [6] | 游弋晖, 宋春晖, 陈 曦, 姚晓宏, 柯俊羽, 杜 群, 宋 宁, 李燕舞. 沉默信息调节因子6基因敲除模型小鼠肠黏膜形态及转录组学的特征性变化[J]. 中国组织工程研究, 2022, 26(32): 5119-5125. |
| [7] | 刘 超, 曾昭穆, 温稀超, 吴文松, 孙程圆, 郑克彬. 外泌体非编码RNA在胶质瘤发生发展中的作用及其临床应用前景[J]. 中国组织工程研究, 2022, 26(24): 3928-3936. |
| [8] | 冯 辰, 周骥平, 许晓东, 姜亚妮, 史宏灿, 赵国琦. 3D打印生物材料微小力学性能自动测量系统的设计[J]. 中国组织工程研究, 2022, 26(21): 3306-3311. |
| [9] | 苑 龙, 李 森, 卞继超, 李万祥, 王国栋. 3D生物打印生物墨水灭菌技术的合理选择与应用[J]. 中国组织工程研究, 2022, 26(18): 2914-2921. |
| [10] | 张秀梅, 翟运开, 赵 杰, 赵 萌. 类器官模型国内外数据库近10年文献研究热点分析[J]. 中国组织工程研究, 2021, 25(8): 1249-1255. |
| [11] | 焦 慧, 张一宁, 宋雨晴, 林 宇, 王秀丽. 乳腺癌类器官研究进展及临床应用前景[J]. 中国组织工程研究, 2021, 25(7): 1122-1128. |
| [12] | 杨振, 李浩, 付力伟, 高仓健, 姜双鹏, 王福鑫, 苑志国, 孙志强, 查康康, 田广招, 曹福洋, 眭翔, 刘舒云, 郭全义. 3D生物打印负载转化生长因子β3的软骨复合支架[J]. 中国组织工程研究, 2021, 25(34): 5445-5452. |
| [13] | 刘 琦, 姚 茜, 韦正波, 谢 莹. 类器官培养基主要成分的作用机制及潜在功能[J]. 中国组织工程研究, 2021, 25(31): 5072-5078. |
| [14] | . 载血小板衍生生长因子3D生物打印半月板支架的制备流程[J]. 中国组织工程研究, 2021, 25(28): 4465-4472. |
| [15] | 冯紫伊, 梁珊珊, 于炜婷, 王若雨. 患者来源肿瘤类器官的培养与研究及应用[J]. 中国组织工程研究, 2021, 25(25): 4082-4088. |
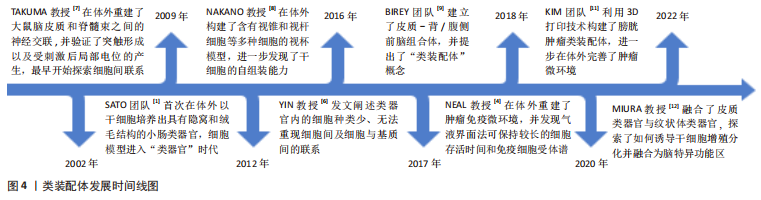
早在2002年,TAKUMA教授[7]便在体外探索了大鼠脑皮质和脊髓束之间神经交联的重建,并刺激它们产生了局部电位,初次在体外探索了细胞间的联系。直到2012年,NAKANO等[8]在体外培养出含有人类视锥和视杆等多种细胞的视杯模型,进一步认识到细胞之间互作的重要性。在类器官发展迅速之时,YIN教授[6]于2016年发文阐述类器官存在细胞种类少、无法在体外完全重现细胞间及细胞与基质间的联系的缺点,这进一步激发了学者们在体外探索多细胞模型及微环境重建的热情。至2017年,BIREY团队[9]首次提出了“类装配体(Assembloids)”概念[10],即由多种类型细胞组成、具有空间结构的类器官,见图1。在该项研究中,研究者建立了皮质-背/腹侧前脑组合体,二者之间实现了谷氨酸或γ-氨基丁酸能神经元的交联。随后,NEAL教授[4]在体外创建了胆管癌、脑神经鞘瘤和唾液多形性瘤等含有免疫微环境的肿瘤类装配体,为肿瘤免疫疗法及精准医疗的进一步发展提供了开创性思路。
 近几年,KIM团队[11]在此概念基础上将膀胱肿瘤类器官和成纤维细胞、内皮细胞等间质细胞通过3D生物打印技术构建出膀胱肿瘤类装配体,也实现了肿瘤免疫微环境的重塑。直到2022年,有学者融合了皮质类器官与纹状体类器官,探索了如何诱导干细胞增殖分化并融合为脑特异功能区,以及远程神经元连接的方式[12]。目前,已有多种器官的类装配体被成功构建,如肠道[13]、大脑[14]、心脏[15-17]、肝脏[18]、胰腺[19-20]、肾脏[18]、膀胱[21]、胃癌[22]、视网膜和骨骼等[23-24]。类装配体是类器官的延续和完善,不仅保留了类器官在组织学、遗传学和生理病理特点等方面的优点,还具备脉管系统雏形及复杂的间质细胞成分,并实现细胞间、细胞-间质之间的相互联系,可重塑体内微环境,较类器官更趋近于人体组织,是目前最为新颖且潜力巨大的细胞模型,对于基础研究及临床实验来说,是更为理想的平台,在精准医疗的发展进程中意义重大。
近几年,KIM团队[11]在此概念基础上将膀胱肿瘤类器官和成纤维细胞、内皮细胞等间质细胞通过3D生物打印技术构建出膀胱肿瘤类装配体,也实现了肿瘤免疫微环境的重塑。直到2022年,有学者融合了皮质类器官与纹状体类器官,探索了如何诱导干细胞增殖分化并融合为脑特异功能区,以及远程神经元连接的方式[12]。目前,已有多种器官的类装配体被成功构建,如肠道[13]、大脑[14]、心脏[15-17]、肝脏[18]、胰腺[19-20]、肾脏[18]、膀胱[21]、胃癌[22]、视网膜和骨骼等[23-24]。类装配体是类器官的延续和完善,不仅保留了类器官在组织学、遗传学和生理病理特点等方面的优点,还具备脉管系统雏形及复杂的间质细胞成分,并实现细胞间、细胞-间质之间的相互联系,可重塑体内微环境,较类器官更趋近于人体组织,是目前最为新颖且潜力巨大的细胞模型,对于基础研究及临床实验来说,是更为理想的平台,在精准医疗的发展进程中意义重大。目前,虽然类装配体所重塑的体内微环境更具有仿真性,相较其他细胞模型来说,在细胞及组织的发育机制、疾病的病理机制、药物筛选及研发等研究中具有无可比拟的优势,但由于其处于初步发展阶段,关于类装配体的构建方法、应用及局限性等方面均无全面的报道。因此,文章在总结前人相关研究的基础上,对类装配体的构建方法、目前应用和存在的局限性等进行综述,进一步讨论类装配体构建方法的优劣势、适用方向和前景,以期将来为进一步改进类装配体、构建具有完整调控中心且更仿真的模型奠定基础。 中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
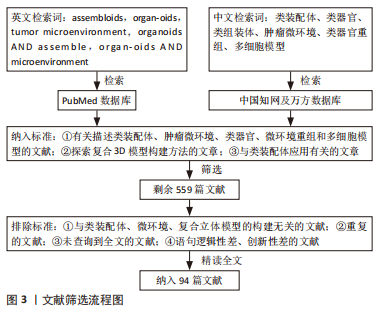
1.1.1 检索人及检索时间 第一作者在2022年12月进行检索。
1.1.2 检索文献时限 2002-2022年期间发表的相关文献,重点检索2012-2022年期间发表的文章。
1.1.3 检索数据库 PubMed、中国知网和万方数据库。
1.1.4 检索词 以“assembloids,organoids,tumor microenvironment,organoids AND assemble,organoids AND microenvironment”为英文检索词检索;以“类装配体、类器官、类组装体、肿瘤微环境、类器官和重组、多细胞模型”为中文检索词检索。
1.1.5 检索文献类型 研究原著和综述。
1.1.6 手工检索情况 未进行手工检索。
1.1.7 检索策略 以PubMed数据库检索策略为例,见图2。

1.2 入组标准
1.2.1 纳入标准 ①有关描述类装配体、肿瘤微环境、类器官、微环境重组和多细胞模型的文献;②探索复合3D模型构建方法的文章;③有关类装配体应用方面的文章。
1.2.2 排除标准 ①与类装配体、微环境、复合立体模型构建无关的文献;②重复的文献;③未查询到全文的文献;④文章语句逻辑性差创新性差的文献。
1.3 数据的提取 按照入选标准对检索结果中与类组装体、3D细胞复合模型、多细胞模型、类器官、微环境、立体模型及微环境重构相关的文章初步阅读,筛选出559篇文献。按照排除标准对上述筛选出的文章进行精读,最终筛选出94篇进行综述,其中来自PubMed数据库93篇,来自中国知网数据库1篇,文献筛选流程见图3。
3.2 作者综述区别于他人他篇的特点 文章以类装配体构建方法发展时间轴为线索,从起始细胞的不同以及细胞培养方法的区别两个方面对类装配体构建方法分别进行了阐述,并详细介绍了目前较先进的器官芯片及3D打印技术在类装配体研究中的应用,有助于深入了解类装配体构建方法的现状。另外,文章还对类装配体的应用前景及存在的局限性进行了系统阐释,讨论了不同构建方法在不同研究领域中的优势,为未来进一步完善类装配体,构建更为精准的仿真器官提供指导。
3.3 综述的重要意义和局限性 类装配体是目前最具潜力的体外细胞模型,对生物科学研究具有重要意义,但是它构建的方法较复杂,完全掌握该技术的研究团队仍不多。文章从类装配体的构建方法、应用前景以及局限性等方面对其研究现状进行系统阐述,帮助后续研究人员进一步认识、掌握类装配体的构建方法,为未来完善类装配体的构建方式及应用等研究奠定基础。但目前类装配体处于发展初期,相关研究尚不够完善,使得该综述存在一些局限性:①由于体内器官发育机制具有复杂多样性,各种细胞的分化及互作需要不同的细胞因子,且目前尚无统一且规范的类装配体构建流程,因此文章未能对其中的细胞分化、互作顺序进行总结,且对于类装配体的发育生长调控的研究较少,文章未能进行全面的总结;②鲜有文献报道脉管化类装配体中脉管形成过程中各细胞之间的具体机制,以及类装配体的脉管网络是否与体内脉管功能相似也未进行深入探究,因此,这也是文章尚未详细介绍的方面。
3.4 课题专家组对未来的建议 ①通过完善构建方法,缩小类装配体细胞与体内细胞在分化过程中产生的差异,提高类装配体的仿真程度和应用效率,如结合各种构建方式和培养方法,探索更完善的血管化类装配体;在类装配体培养之初引入对应器官特异的信号中心,如Shh蛋白梯度系统[86],指导类装配体的各种细胞有序且自发地分化、组装成“器官”。②探索了解类装配体各细胞间的相互作用机制及信号转导机制,通过一定的干预处理,调控类装配体发育进程。③可尝试将多器官的类装配体进行共培养,创建人体“微系统组合”,可更准确地反映生理、病理和药物作用机制,为精准医疗提供更可靠的依据。 中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
 #br#
#br#
文题释义:
类装配体:是由多种类型细胞组成、具有空间结构的类器官,亦称为类组装体,它可比传统类器官更好地复制体内微环境、保持细胞的遗传物质和组织特点。自体组装:是指由干细胞增殖聚集,并被诱导分化形成含有多种细胞的类装配体的过程。
直接组装:指在体外直接将多种分化成熟的细胞按一定比例共培养,组装为类装配体的过程。
混合组装:指由干细胞形成的类器官或细胞球与分化成熟的不同细胞共培养,形成类装配体的过程。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
虽然“类器官(organoids)”问世后广受好评,但其因缺乏复杂的间质成分,而无法完全重现细胞与细胞、细胞与基质间的互作关系,因此构建具有复杂细胞类型和间质成分的“升级版类器官-类装配体(assembloids)”在生命科学研究中显得尤为重要。近年来,已有许多学者探索了各种类装配体的构建方法,并有许多研究证实类装配体可弥补类器官的上述缺点,但处于发展初期的类装配体构建方式种类繁多,更无统一标准,因此,对其构建方法、应用领域和局限性进行归纳总结,为其后续的发展提供思路很有必要。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||