| [1] Uritani D, Fukumoto T, Myodo T, et al. The association between toe grip strength and osteoarthritis of the knee in Japanese women: a multicenter cross-sectional study. PLoS One. 2017. doi: 10.1371/journal.pone.0186454. [2] Favero M, El-Hadi H, Belluzzi E, et al. Infrapatellar fat pad features in osteoarthritis: a histopathological and molecular study. Rheumatology (Oxford). 2017. doi: 10.1093/rheumatology/kex287.[3] Kuttapitiya A, Assi L, Laing K, et al. Microarray analysis of bone marrow lesions in osteoarthritis demonstrates upregulation of genes implicated in osteochondral turnover, neurogenesis and inflammation. Ann Rheum Dis. 2017. doi: 10.1136/annrheumdis-2017-211396.[4] Frank RM, Della Valle CJ, Plummer DR, et al. Does prior cartilage restoration impact outcomes following knee arthroplasty? Orthop Clin North Am. 2017. doi: 10.1016/j.ocl.2017.03.001. [5] McDaniel D, Tilton E, Dominick K, et al. Histological characteristics of knee menisci in patients with osteoarthritis. Clin Anat. 2017. doi: 10.1002/ca.22920. [6] Chang NJ, Lee KW, Chu CJ, et al. A Preclinical assessment of early continuous passive motion and treadmill therapeutic exercises for generating chondroprotective effects after anterior cruciate ligament rupture. Am J Sports Med. 2017. doi: 10.1177/0363546517704847. [7] Ha CW, Park YB, Kyung HS, et al. Gastrointestinal safety and efficacy of long-term GCSB-5 use in patients with osteoarthritis: a 24-week, multicenter study. J Ethnopharmacol. 2016;189:310-318. [8] Zhang XL, Yang J, Yang L, et al. Efficacy and safety of zhuanggu joint capsules in combination with celecoxib in knee osteoarthritis: a multi-center, randomized, double-blind, double-dummy, and parallel controlled trial. Chin Med J (Engl). 2016;129(8):891-897.[9] da Costa BR, Reichenbach S, Keller N, et al. RETRACTED: Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet. 2016;387:2093-2105. [10] Lee T, Lu N, Felson DT, et al. Use of non-steroidal anti-inflammatory drugs correlates with the risk of venous thromboembolism in knee osteoarthritis patients: a UK population-based case-control study. Rheumatology (Oxford). 2016;55(6):1099-1105. [11] Rezende MU, Hernandez AJ, Oliveira CR, et al. Experimental osteoarthritis model by means of medial meniscectomy in rats and effects of diacerein administration and hyaluronic acid injection. Sao Paulo Med J. 2015;133:4-12. [12] Fidelix TS, Macedo CR, Maxwell LJ, et al. Diacerein for osteoarthritis. Cochrane Database Syst Rev. 2014. doi: 10.1002/14651858.CD005117.pub3. [13] Jain A, Mishra SK, Vuddanda PR, et al. Targeting of diacerein loaded lipid nanoparticles to intra-articular cartilage using chondroitin sulfate as homing carrier for treatment of osteoarthritis in rats. Nanomedicine. 2014;10:1031-1040. [14] McAlindon TE, Bannuru RR, Sullivan MC, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2014;22:363-88. [15] 中华医学会骨科学分会.骨关节炎诊治指南(2007年版)[J].中华骨科杂志,2007,27(10):793-796.[16] Kooistra BW, Spraque S, Bhandari M, et al. Outcomes assessment in fracture healing trials: a primer. J Orthop Trauma. 2010;24 SupplⅠ:S71-S75. [17] Bellamy N, Buchanan WW, Goldsmith CH, et al. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833-1840.[18] 陈琦,何斌,王云华,等.塞来昔布联合双醋瑞因治疗膝骨关节炎的疗效观察[J].实用骨科杂志,2010,16(12):894-896.[19] Liu GB, Zhang GP, Ren QY, et al. Classification of ankle injury on radiography and magnetic resonance imaging: study protocol for a retrospective, self-controlled, clinical trial with 3-month followup. Clin Transl Orthop. 2016;1:170-176.[20] Chen Y, Qu S, Ma G, Meng JH, Ni XL. Femoral nerve block prevents deep venous thrombosis of the lower extremity after knee arthroplasty: a single-center randomized controlled trial. Clin Transl Orthop. 2016;1:1-5. |
.jpg) 文题释义:
塞来昔布:通过选择性抑制环氧化酶2来抑制前列腺素生成,达到抗炎症、镇痛的效果,由于它不会抑制具有胃肠道保护作用的生理酶——环氧化酶1,其胃肠道不良反应风险明显低于传统非类固醇抗炎镇痛药。
双醋瑞因:可诱导软骨生成,具有止痛、抗炎及退热作用,也可显著改善骨关节炎患者的关节功能,延缓病程,减轻疼痛,提高患者的生活质量,具有较好的安全性,临床主要用于治疗骨关节炎而发挥抗菌作用。
文题释义:
塞来昔布:通过选择性抑制环氧化酶2来抑制前列腺素生成,达到抗炎症、镇痛的效果,由于它不会抑制具有胃肠道保护作用的生理酶——环氧化酶1,其胃肠道不良反应风险明显低于传统非类固醇抗炎镇痛药。
双醋瑞因:可诱导软骨生成,具有止痛、抗炎及退热作用,也可显著改善骨关节炎患者的关节功能,延缓病程,减轻疼痛,提高患者的生活质量,具有较好的安全性,临床主要用于治疗骨关节炎而发挥抗菌作用。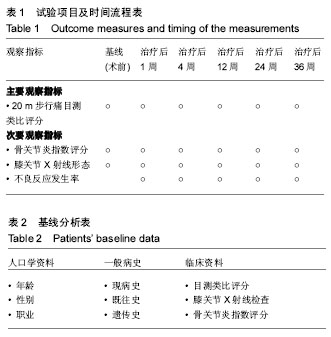
.jpg)
.jpg) 文题释义:
塞来昔布:通过选择性抑制环氧化酶2来抑制前列腺素生成,达到抗炎症、镇痛的效果,由于它不会抑制具有胃肠道保护作用的生理酶——环氧化酶1,其胃肠道不良反应风险明显低于传统非类固醇抗炎镇痛药。
双醋瑞因:可诱导软骨生成,具有止痛、抗炎及退热作用,也可显著改善骨关节炎患者的关节功能,延缓病程,减轻疼痛,提高患者的生活质量,具有较好的安全性,临床主要用于治疗骨关节炎而发挥抗菌作用。
文题释义:
塞来昔布:通过选择性抑制环氧化酶2来抑制前列腺素生成,达到抗炎症、镇痛的效果,由于它不会抑制具有胃肠道保护作用的生理酶——环氧化酶1,其胃肠道不良反应风险明显低于传统非类固醇抗炎镇痛药。
双醋瑞因:可诱导软骨生成,具有止痛、抗炎及退热作用,也可显著改善骨关节炎患者的关节功能,延缓病程,减轻疼痛,提高患者的生活质量,具有较好的安全性,临床主要用于治疗骨关节炎而发挥抗菌作用。