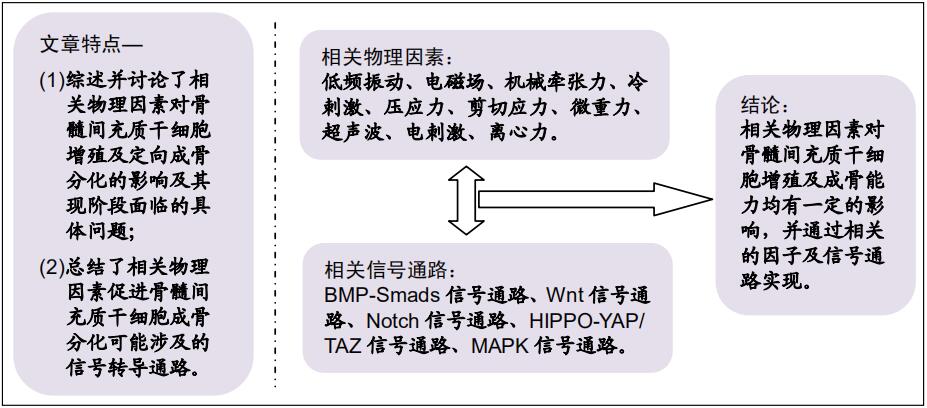
|
[1] NEUHUBER B, SWANGER SA, HOWARD L, et al. Effects of plating density and culture time on bone marrow stromal cell characteristics. Exp Hematol. 2008;36(9):1176-1185.
[2] WULF GG, JACKSON KA, GOODELL MA. Somatic stem cell plasticity: current evidence and emerging concepts. Exp Hematol. 2001;29(12): 1361-1370.
[3] DOMINICI M, LE BLANC K, MUELLER I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006; 8(4):315-317.
[4] DOĞAN A, DEMIRCI S, BAYIR Y, et al. Boron containing poly-(lactide-co-glycolide) (PLGA) scaffolds for bone tissue engineering. Mater Sci Eng C Mater Biol Appl. 2014;44:246-253.
[5] WEI B, YAO Q, GUO Y, et al. Three-dimensional polycaprolactone- hydroxyapatite scaffolds combined with bone marrow cells for cartilage tissue engineering. J Biomater Appl. 2015;30(2):160-170.
[6] VEGA A, MARTÍN-FERRERO MA, DEL CANTO F, et al. Treatment of Knee Osteoarthritis With Allogeneic Bone Marrow Mesenchymal Stem Cells: A Randomized Controlled Trial. Transplantation. 2015;99(8): 1681-1690.
[7] FU Q, TANG NN, ZHANG Q, et al. Preclinical Study of Cell Therapy for Osteonecrosis of the Femoral Head with Allogenic Peripheral Blood-Derived Mesenchymal Stem Cells. Yonsei Med J. 2016;57(4): 1006-1015.
[8] LI X, ZHANG Y, SONG B, et al. Experimental Application of Bone Marrow Mesenchymal Stem Cells for the Repair of Intervertebral Disc Annulus Fibrosus. Med Sci Monit. 2016;22:4426-4430.
[9] ZHANG J, LI L. BMP signaling and stem cell regulation. Dev Biol. 2005; 284(1):1-11.
[10] SHI Y, MASSAGUÉ J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113(6):685-700.
[11] VARGA AC, WRANA JL. The disparate role of BMP in stem cell biology. Oncogene. 2005;24(37):5713-5721.
[12] LI S, LU K, WANG J, et al. Ubiquitin ligase Smurf1 targets TRAF family proteins for ubiquitination and degradation. Mol Cell Biochem. 2010; 338(1-2):11-17.
[13] LI E, ZHANG J, YUAN T, et al. MiR-143 suppresses osteogenic differentiation by targeting Osterix. Mol Cell Biochem. 2014;390(1-2): 69-74.
[14] BAGLÌO SR, DEVESCOVI V, GRANCHI D, et al. MicroRNA expression profiling of human bone marrow mesenchymal stem cells during osteogenic differentiation reveals Osterix regulation by miR-31. Gene. 2013;527(1):321-331.
[15] SHI YC, WORTON L, ESTEBAN L, et al. Effects of continuous activation of vitamin D and Wnt response pathways on osteoblastic proliferation and differentiation. Bone. 2007;41(1):87-96.
[16] GLASS DA 2ND, BIALEK P, AHN JD, et al. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell. 2005;8(5):751-764.
[17] MONROE DG, MCGEE-LAWRENCE ME, OURSLER MJ, et al. Update on Wnt signaling in bone cell biology and bone disease. Gene. 2012;492(1):1-18.
[18] JAVAHERI B, STERN AR, LARA N, et al. Deletion of a single β-catenin allele in osteocytes abolishes the bone anabolic response to loading. J Bone Miner Res. 2014;29(3):705-715.
[19] MACSAI CE, FOSTER BK, XIAN CJ. Roles of Wnt signalling in bone growth, remodelling, skeletal disorders and fracture repair. J Cell Physiol. 2008;215(3):578-587.
[20] LAI EC. Notch signaling: control of cell communication and cell fate. Development. 2004;131(5):965-973.
[21] ENGIN F, YAO Z, YANG T, et al. Dimorphic effects of Notch signaling in bone homeostasis. Nat Med. 2008;14(3):299-305.
[22] WATANABE K, IKEDA K. Osteoblast differentiation and bone formation. Nihon Rinsho. 2009;67(5):879-886.
[23] 顾客,田慧,李傲楠,等.HIPPO-YAP/TAZ信号通路与干细胞分化相关信号通路交叉作用的研究进展[J].牙体牙髓牙周病学杂志,2018,28(11):667-672.
[24] MURILLO-GARZÓN V, KYPTA R. WNT signalling in prostate cancer. Nat Rev Urol. 2017;14(11):683-696.
[25] HUANG SM, MISHINA YM, LIU S, et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461(7264): 614-620.
[26] ZHANG P, WU Y, JIANG Z, et al. Osteogenic response of mesenchymal stem cells to continuous mechanical strain is dependent on ERK1/2-Runx2 signaling. Int J Mol Med. 2012;29(6):1083-1089.
[27] TANAKA SM, ALAM IM, TURNER CH. Stochastic resonance in osteogenic response to mechanical loading. FASEB J. 2003;17(2): 313-314.
[28] HU N, FENG C, JIANG Y, et al. Regulative Effect of Mir-205 on Osteogenic Differentiation of Bone Mesenchymal Stem Cells (BMSCs): Possible Role of SATB2/Runx2 and ERK/MAPK Pathway. Int J Mol Sci. 2015;16(5):10491-10506.
[29] KING LK, ALMEIDA QJ, AHONEN H. Short-term effects of vibration therapy on motor impairments in Parkinson's disease. NeuroRehabilitation. 2009;25(4):297-306.
[30] HOTAMISLIGIL GS, DAVIS RJ. Cell Signaling and Stress Responses. Cold Spring Harb Perspect Biol. 2016;8(10). pii: a006072.
[31] FAHY N, ALINI M, STODDART MJ. Mechanical stimulation of mesenchymal stem cells: Implications for cartilage tissue engineering. J Orthop Res. 2018;36(1):52-63.
[32] HONG JH, HWANG ES, MCMANUS MT, et al. TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science. 2005; 309(5737):1074-1078.
[33] DUPONT S, MORSUT L, ARAGONA M, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474(7350):179-183.
[34] TANG Y, ROWE RG, BOTVINICK EL, et al. MT1-MMP-dependent control of skeletal stem cell commitment via a β1-integrin/YAP/TAZ signaling axis. Dev Cell. 2013;25(4):402-416.
[35] KIM IS, SONG YM, LEE B, et al. Human mesenchymal stromal cells are mechanosensitive to vibration stimuli. J Dent Res. 2012;91(12): 1135-1140.
[36] BOYCE BF, XING L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys. 2008;473(2): 139-146.
[37] CECCARELLI G, BENEDETTI L, GALLI D, et al. Low-amplitude high frequency vibration down-regulates myostatin and atrogin-1 expression, two components of the atrophy pathway in muscle cells. J Tissue Eng Regen Med. 2014;8(5):396-406.
[38] DAISH C, BLANCHARD R, DUCHI S, et al. Design, Fabrication and Validation of a Precursor Pulsed Electromagnetic Field Device for Bone Fracture Repair. Conf Proc IEEE Eng Med Biol Soc. 2018;2018: 4166-4169.
[39] ROSS CL, SIRIWARDANE M, ALMEIDA-PORADA G, et al. The effect of low-frequency electromagnetic field on human bone marrow stem/progenitor cell differentiation. Stem Cell Res. 2015;15(1):96-108.
[40] YONG Y, MING ZD, FENG L, et al. Electromagnetic fields promote osteogenesis of rat mesenchymal stem cells through the PKA and ERK1/2 pathways. J Tissue Eng Regen Med. 2016;10(10):E537-E545.
[41] 韦盛,杨勇,赵东明,等.白细胞介素-1β和电磁场对大鼠骨髓间充质干细胞成骨分化的影响[J].骨科,2019,10(4):335-339.
[42] BURR DB, MILGROM C, FYHRIE D, et al. In vivo measurement of human tibial strains during vigorous activity. Bone. 1996;18(5): 405-410.
[43] QI MC, ZOU SJ, HAN LC, et al. Expression of bone-related genes in bone marrow MSCs after cyclic mechanical strain: implications for distraction osteogenesis. Int J Oral Sci. 2009;1(3):143-150.
[44] KOIKE M, SHIMOKAWA H, KANNO Z, et al. Effects of mechanical strain on proliferation and differentiation of bone marrow stromal cell line ST2. J Bone Miner Metab. 2005;23(3):219-225.
[45] SIMMONS CA, MATLIS S, THORNTON AJ, et al. Cyclic strain enhances matrix mineralization by adult human mesenchymal stem cells via the extracellular signal-regulated kinase (ERK1/2) signaling pathway. J Biomech. 2003;36(8):1087-1096.
[46] ZHANG P, WU Y, DAI Q, et al. p38-MAPK signaling pathway is not involved in osteogenic differentiation during early response of mesenchymal stem cells to continuous mechanical strain. Mol Cell Biochem. 2013;378(1-2):19-28.
[47] CHEN D, LI Y, ZHOU Z, et al. Synergistic inhibition of Wnt pathway by HIF-1α and osteoblast-specific transcription factor osterix (Osx) in osteoblasts. PLoS One. 2012;7(12):e52948.
[48] KANG KS, LEE SJ, LEE HS, et al. Effects of combined mechanical stimulation on the proliferation and differentiation of pre-osteoblasts. Exp Mol Med. 2011;43(6):367-373.
[49] 薛令法,张岱尊,肖文林,等.机械牵张力促进小鼠骨髓间充质干细胞的成骨向分化[J].国际口腔医学杂志,2017,44(6):679-685.
[50] PUTRI M, SYAMSUNARNO MR, ISO T, et al. CD36 is indispensable for thermogenesis under conditions of fasting and cold stress. Biochem Biophys Res Commun. 2015;457(4):520-525.
[51] 罗依,余勤,丁伟,等低温冻存对成人骨髓间充质干细胞生物学特性的影响[J].中国病理生理杂志,2006,22(2): 384-387.
[52] 聂义珍,闫朝岐,付红梅,等.冷刺激对骨髓间充质干细胞增殖及成骨分化的影响[J].中华骨质疏松和骨矿盐疾病杂志, 2018,11(6):577-583.
[53] SODEK J, CHEN J, NAGATA T, et al. Regulation of osteopontin expression in osteoblasts. Ann N Y Acad Sci. 1995;760:223-241.
[54] HUANG C, OGAWA R. Effect of hydrostatic pressure on bone regeneration using human mesenchymal stem cells. Tissue Eng Part A. 2012;18(19-20):2106-2113.
[55] STAVENSCHI E, CORRIGAN MA, JOHNSON GP, et al. Physiological cyclic hydrostatic pressure induces osteogenic lineage commitment of human bone marrow stem cells: a systematic study. Stem Cell Res Ther. 2018;9(1):276.
[56] 姜喜亮,朱晓文,胡静,等.整合素信号通路在大鼠骨髓间充质干细胞牵张中的作用和转导机制[J].中国口腔颌面外科杂志, 2015,13(3):193-199.
[57] LIU J, ZHAO Z, ZOU L, et al. Pressure-loaded MSCs during early osteodifferentiation promote osteoclastogenesis by increase of RANKL/OPG ratio. Ann Biomed Eng. 2009;37(4):794-802.
[58] LIU L, YU B, CHEN J, et al. Different effects of intermittent and continuous fluid shear stresses on osteogenic differentiation of human mesenchymal stem cells. Biomech Model Mechanobiol. 2012;11(3-4): 391-401.
[59] VETSCH JR, BETTS DC, MÜLLER R, et al. Flow velocity-driven differentiation of human mesenchymal stromal cells in silk fibroin scaffolds: A combined experimental and computational approach. PLoS One. 2017;12(7):e0180781.
[60] HU K, SUN H, GUI B, et al. TRPV4 functions in flow shear stress induced early osteogenic differentiation of human bone marrow mesenchymal stem cells. Biomed Pharmacother. 2017;91:841-848.
[61] BECQUART P, CRUEL M, HOC T, et al. Human mesenchymal stem cell responses to hydrostatic pressure and shear stress. Eur Cell Mater. 2016;31:160-173.
[62] 李煜,孙明林,张新昌,等.微重力对骨髓间充质干细胞向成骨细胞分化影响的研究[J].国际生物医学工程杂志, 2015,38(5):257-261.
[63] MAO X, CHEN Z, LUO Q, et al. Simulated microgravity inhibits the migration of mesenchymal stem cells by remodeling actin cytoskeleton and increasing cell stiffness. Cytotechnology. 2016;68(6):2235-2243.
[64] LI L, ZHANG C, CHEN JL, et al. Effects of simulated microgravity on the expression profiles of RNA during osteogenic differentiation of human bone marrow mesenchymal stem cells. Cell Prolif. 2019; 52(2):e12539.
[65] 杨先炯,毛新建,罗庆,等.Wnt3a/β-catenin信号通路调节模拟微重力诱导的骨髓间充质干细胞增殖抑制[J].空间科学学报, 2016,36(1):63-70.
[66] 杜静静,谭信.模拟微重力下骨髓间充质干细胞成骨分化潜能及分子机理研究[J].生命科学仪器,2018,16(2):38-43.
[67] WARDEN SJ, BENNELL KL, MCMEEKEN JM, et al. Acceleration of fresh fracture repair using the sonic accelerated fracture healing system (SAFHS): a review. Calcif Tissue Int. 2000;66(2):157-163.
[68] 李亚明.低强度脉冲超声促进大鼠牙囊细胞、骨髓间充质干细胞成骨分化的初步研究[D].重庆:重庆医科大学,2016.
[69] 王前,郑磊.电刺激促进成骨细胞粘附特性的实验研究[J].暨南大学学报(自然科学与医学版),2000,21(1):46-49.
[70] TSAI MT, LI WJ, TUAN RS, et al. Modulation of osteogenesis in human mesenchymal stem cells by specific pulsed electromagnetic field stimulation. J Orthop Res. 2009;27(9):1169-7114.
[71] 蒋斌,郑明辉,张斌,等.三维载体培养下离心力对骨髓间充质干细胞增殖的影响[J].解剖学杂志,2018,41(1):1-4.
[72] 段峰,关键,杨红岩,等.机械离心力对成骨细胞骨形态发生蛋白信号通路中Runx-2 mRNA的影响[J].中国组织工程研究,2014,18(33):5305-5309.
|