1.1 试验目的 比较儿童口腔科常用材料MTA、氢氧化钙制剂、Biodentine及TheraCal LC在乳牙牙髓保存术中的临床疗效,观察几种生物材料的作用下牙本质桥形成的速度、质量,并评估治疗失败的原因,为临床医生在选择乳牙牙髓保存术材料时提供参考,提高乳牙牙髓保存术的成功率,降低并发症的产生。
1.2 试验方法选择及理由 ①首次系统性的比较不同生物材料作为盖髓剂在3种活髓保存术中的临床应用疗效;②在生物材料的选择方面,分析经典生物材料MTA、氢氧化钙制剂与最新型生物材料Biodentine与TheraCalLC作为盖髓剂的临床疗效;③除了生物材料的选择,本课题还注重乳牙活髓保存术结束后冠修复的问题,建议选取最优的盖髓剂的同时,也要考虑使用预成冠完成最优的冠方封闭,两者结合才能获取最高的成功率和远期临床疗效。
1.3 减少、避免偏倚的措施 ①患者的入组严格按照纳入排除标准筛选;②应用计算机随机数字法进行随机分组;③所有的乳牙活髓保存术均由同一组医师进行,采用同一品牌的盖髓材料;④数据测量工作由一个对本研究不知情的审查员独立进行;⑤采用了盲法:评估者(包括临床检查和影像学评价)不清楚患者的分组情况;患者也不知道自己的分组情况;数据的统计分析由不清楚分组的专业统计人员进行。
1.4 试验设计类型 前瞻性、单中心、随机对照、双盲临床试验研究。
1.5 试验完成地点 中国,四川省,成都市妇幼儿童中心医院。
1.6 受试者选择
1.6.1 纳入标准
实施间接盖髓术或直接盖髓术患儿的纳入标准:①患儿均来自于成都市妇女儿童中心;②患儿年龄4-7岁;③患牙为牙周健康的牙合面或邻面深龋;④患牙无自发痛史、牙根长度超过2/3、无根尖周病变和窦道;⑤患儿父母或监护人对临床试验方案了解并同意参与试验,并签署书面“知情同意书”。
实施间牙髓切断术患儿的纳入标准:①患儿均来自于成都市妇女儿童中心;②患儿年龄3-8岁;③患牙为被诊断为深龋的下颌乳磨牙;④患牙均处于牙根稳定期;⑤患儿父母或监护人对临床试验方案了解并同意参与试验,并签署书面“知情同意书”。
空白对照牙的纳入标准:①选取患牙对侧同名正常牙作为空白对照;②临床检查无龋损;③X射线片显示根分叉处、根尖周范围无暗影,牙根亦处于稳定期;④患儿父母或监护人对临床试验方案了解并同意参与试验,并签署书面“知情同意书”。
1.6.2 排除标准 牙齿有明确牙髓病变、牙内吸收、牙髓钙化、根尖或根分叉病变者被排除。
1.6.3 入组时间 病例的收集时间是2016年9月至2017 年6月。
1.6.4 每位受试者的预期参与持续时间 每位受试者随访至术后12个月,因此其预期参与持续时间为2年。
1.6.5 临床试验所需的受试者数量 预期纳入间接盖髓术组或直接盖髓术组的患儿各60例,纳入牙髓切断术组45例。
1.7 招募 在成都市妇女儿童中心口腔科门诊发放试验招募宣传单,同时医生在门诊收治的诊断为乳牙深龋、需行乳牙活髓保存术的患儿家属群中宣传此次试验的招募条件,有感兴趣的患儿家属或监护人可通过主治医生,以电话、e-mail或微信的形式联系项目负责人,招募至研究组进行筛选。符合要求的患儿在家属或监护人了解试验内容,签署试验知情同意书后入组。
1.8 试验分组
1.8.1 产生序列分配的方法 应用计算机随机数字法进行随机分组,进行间接盖髓术的60例患者随机分为4组,每组15例;进行直接盖髓术的60例患者随机分为4组,每组15例;进行牙髓切断术的45例患儿60颗牙,随机分为4组,每组15颗牙。
1.8.2 分配隐藏及实施 计算机产生的各组随机名单装入密封的不透明的信封中,由指定的不参与手术干预的课题组成员保存。
1.8.3 盲法 评估者(包括临床检查和影像学评价)不清楚患者的分组情况;患者也不知道自己的分组情况;数据的统计分析由不清楚分组的专业统计人员进行。
1.9 干预措施 3种乳牙活髓保存术均由同一位医师进行。
1.9.1 不同生物制剂在间接盖髓术中的应用 60例患儿根据所使用的材料不同将患牙牙齿随机分成4组:MTA组、氢氧化钙组、Biodentine组和TheraCal组,每组15例。
治疗程序:局麻后,上橡皮帐隔离患牙,使用大球钻低速去腐,去净龋洞侧壁和洞缘的腐质,用视诊和探诊相结合的方法去除洞底软化的牙本质并保留洞底反应性牙本质,腐质去除后,用生理盐水冲洗窝洞10 s,用消毒棉球拭干窝洞。MTA组、Biodentine组使用MTA,Biodentine手工调制,取适量轻轻盖在洞底,上方置一湿润小棉球,ZnOE暂封,45 min后去除暂封和棉球,树脂改良型玻璃离子水门汀垫底,复合树脂修复。氢氧化钙组、TheraCal组直接将氢氧化钙、TheraCal LC推注到牙本质表面,光固化机光照20 s,树脂改良型玻璃离子水门汀垫底,复合树脂修复。充填后拍摄X射线片确定基准值。
1.9.2 不同生物制剂在直接盖髓术中的应用 60例患儿根据所使用的材料不同将患牙牙齿随机分成4组:MTA组、氢氧化钙组、Biodentine组和TheraCal组,每组15例。
治疗程序:程序局麻后,上橡皮帐隔离患牙,使用大球钻低速去腐,去净龋洞侧壁和洞缘的腐质,用视诊和探诊相结合的方法去尽腐质,去龋过程中意外露髓,露髓孔小于1 mm,用生理盐水冲洗窝洞10 s,用消毒棉球拭干窝洞。MTA组、Biodentine组使用MTA,Biodentine手工调制,取适量轻轻盖在洞底,上方置一湿润小棉球,ZnOE暂封,45 min后去除暂封和棉球,树脂改良型玻璃离子水门汀垫底,复合树脂修复。氢氧化钙组、TheraCal组直接将氢氧化钙、TheraCal LC推注到牙本质表面,光固化机光照20 s,树脂改良型玻璃离子水门汀垫底,复合树脂修复。充填后拍摄X射线片确定基准值。
1.9.3 不同生物制剂在牙髓切断术中的应用 45例患儿的60颗患牙被随机分为MTA组15个、氢氧化钙组15个、Biodentine组15个、TheraCal LC组15个, 所有的患牙均选取对侧同名正常牙作为空白对照。
规范化操作流程:上橡皮障,采用STA局麻下去净龋坏牙本质、备洞、开髓、揭髓顶、去冠髓、生理盐水冲洗、止血、根据分组置不同盖髓剂、玻璃离子垫底、SSC修复。
1.10 有效性和安全性评价方法
1.10.1 有效性评价方法
(1)主要评价指标:①直接盖髓术和间接盖髓术后主要评价指标:修复性牙本质形成的厚度,将影像数据统一进行XCP技术处理,扫描并传输到计算机中进行数字分析,以术前基准值为据;②活髓切断术的主要评价指标:临床疗效。成功:患牙无任何自觉症状,叩痛(-),无松动,牙龈色性质正常,X射线片未见根分叉及根尖病变及阴影,牙根无吸收;失败:患者有自觉症状,咬合痛,叩(+),不同程度松动,牙龈颜色红肿或出现瘘管;X射线片根分叉及根尖显示阴影,牙根或存在异常吸收。
(2)次要评价指标:牙根吸收程度评定标准:①零度:无异常牙根吸收;②轻度:牙根吸收占根长1/4以内;③中度:牙根吸收占根长1/4-1/2;④重度:牙根吸收占根长1/2以上。
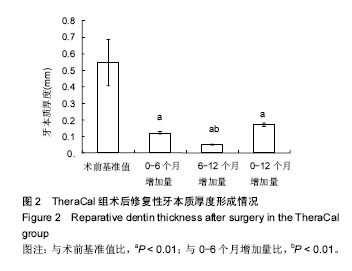
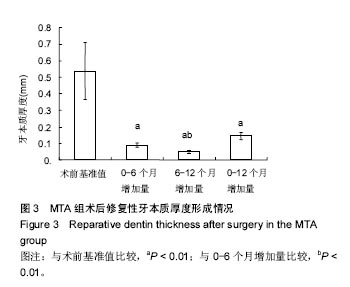
(3)评价、记录和分析有效性参数时间选择 直接盖髓术和间接盖髓术后3,6及12个月拍X射线片进行修复性牙本质形成的厚度测量;活髓切断术后3,6及12个月评估临床疗效。
1.10.2 安全性评价方法
(1)手术并发症及不良反应。
(2)评估治疗失败的原因。
(3)材料宿主反应发生情况。
1.11 试验流程 试验流程图见图1。
.jpg)
1.12 统计学考虑
1.12.1 样本量的计算 结合作者以往经验,试验假设行直接盖髓术和间接盖髓术的各组患儿术后1年修复性牙本质形成的厚度约0.16 mm,设β=0.1,power=90%,显著性水准为双侧α=0.05,采用PASS 11.0软件计算后样本量为每组60例,按15%的脱落率计算,应纳入71例。 最终按入选标准和排除标准筛选后,实际试验组纳入40例,对照组纳入46例患者参加试验。
1.12.2 数据的统计方法 采用SPSS 16.0统计学软件对数据进行统计学处理。显著性水平α定为0.05,P < 0.05为差异有显著性意义。
(1)基线:各组患者性别构成是计数资料,用χ2检验比较组间差异;年龄是计量资料,用one-way ANOVA比较组间差异。
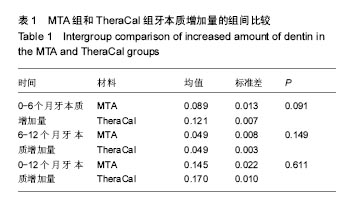
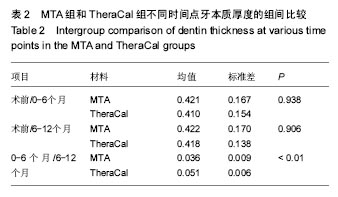
(2)主要疗效指标:修复性牙本质厚度为计量资料,用x±s表示,不同生物材料多组间比较用one-way ANOVA检验,如方差齐两两比较用LSD-t 检验,如方差不齐两两比较用Games-Howell检验。活髓切断术后的临床疗效采用非参数秩和检验比较组间差异。
(3)次要疗效指标:牙根吸收程度采用非参数秩和检验比较组间差异。
(4)安全性评价:并发症及不良反应发生为计数资料,用χ2检验比较组间差异。
1.13 数据管理
1.13.1 病例报告表填写与移交 试验开始前由课题组成员讨论设计统一的病例报告表,并指定2名专门的数据录入人员填写,要求及时并严格遵照方案填写,并保证数据真实完整且与原始资料一致,监察员需定期核对原始数据,所有的数据收集完成后,核对无误,交给项目负责人。
1.13.2 数据录入与修改 所有的资料以双重录入的形式转化为电子版文件。数据只有在监察员、数据录入人员均确认数据有误时方能修改。
1.13.3 数据库锁定 当所有患者的数据收集完成后,核对文件的数据,由项目负责人对数据库进行锁定,之后数据不允许再作变动,该文件需做备份。所有与本次临床试验有关的研究资料均由成都市妇女儿童中心医院保存。
1.13.4 数据处理 将数据库交给专业统计人员进行统计分析,并由统计分析人员写出统计分析报告,交付项目负责人,写出研究报告。
1.14 研究的可行性分析 ①项目组主要成员结构合理、整体素质高,既有儿童口腔医学方面的专家、博士、硕士,也有生物医学工程的专家。项目组成员马小红多年来潜心研究乳牙的牙体牙髓疾病,为课题的完成提供了良好的技术保障。在预实验中,已经摸索出一套行之有效且注重患者舒适度的活髓保存方案;②成都市妇女儿童中心医院是一所集医疗、保健、科研、教学、培训为一体的三级甲等妇女儿童专科医疗与保健机构,担负着成都地区人口中2/3的妇女儿童预防、保健、咨询、医疗等服务,医院设备先进,为课题的实施提供了有力的保障;③徐州市口腔医院为项目的协助单位,徐州市口腔医院是徐州市唯一一所集医疗、教学、科研、预防于一体的口腔专科医院,是淮海经济区规模最大的口腔医院。科室设置齐全,设备先进,在科研、实验等方面为项目的开展给予协助。
1.15 临床试验的伦理问题及知情同意 试验方案经成都市妇女儿童中心医院伦理委员会批准,批准号为20170506。临床试验研究的实施符合《赫尔辛基宣言》和医院对人体研究的相关伦理要求。参与试验的患病个体及其家属为自愿参加,均对试验过程完全知情同意,在充分了解本治疗方案的前提下签署“知情同意书”。
1.16 对不良事件报告的规定
1.16.1 不良事件定义 受试者接受乳牙活髓保存术(包括直接盖髓术、间接盖髓术以及活髓切断术)后出现的不良医学事件,但不一定与治疗有因果关系。
1.16.2 严重不良事件 试验过程中发生需住院治疗、延长住院时间、伤残、危及生命或死亡等事件。
1.16.3 报告程序 医师做好不良事件的记录,包括不良事件的描述,发生时间,终止时间,程度及发作频度,是否需要治疗,如需要,记录给予的治疗。
发生严重不良事件时,除了采取必要的治疗措施以外,应在24 h内如实分别向当地食品药品监督管理局和国家食品药品监督管理局报告,并及时向伦理委员会报告,并在报告上注明日期,严重不良事件处理结束后,应提交受试者情况的后续报道。
1.16.4 不良事件的预测及采取的措施 乳牙活髓保存术常见的并发症是疼痛、感染及材料宿主反应,与材料的生物相容性相关。出现并发症一般是对症处理,如果严重保守治疗不能好转,可拔除患牙。
1.17 保密原则
1.17.1 受试者资料的保密 受试者参加试验及个人试验资料为个人隐私,受试者的全名不会出现在所有记录及文件中,患者姓名用拼音缩写表示。
1.17.2 研究数据的保密 临床试验数据包括书面数据和电子版数据两种形式,电子版数据必须由专门计算机储存,设定开机密码,由资料管理员专人操作和管理;书面数据存放于固定地点并加锁,钥匙由资料管理员和项目负责人保管。
1.18 试验结果发表约定 研究结束后,由主要研究者编写研究总结报告,研究报告中将包括研究目的描述、研究中所使用的方法以及结果和结论,并投稿。
.jpg)
.jpg)
.jpg)