| [1]况花荣,陈同强,刘波兰.体外受精-胚胎移植技术及其进展[J].科技信息,2010(21):555.[2]郑文东.人类辅助生育技术的回顾与展望[J].中国计划生育学志,2002,10(6):378-381.[3]庄广伦,卢光琇,石一复,等.辅助生育技术进展[J].中国实用妇科与产科杂志,2001,17(1):6-31.[4]韩堃,郑源强,石艳春.人类辅助生殖技术研究进展[J].中国妇幼健康研究,2008,19(6):606-609.[5]彭靖,卢大儒.试管婴儿技术的发展与探讨[J].自然杂志, 2010, 32(6):338-343.[6]丁萍,张爱青,孟凡会,等.影响助生殖技术的因素分析[J].青岛大学医学院学报,2001,37(4):277-278.[7]梅志强.人类辅助生殖技术的发展历史与现状[J].中国计划生育学杂志,2008,16(10):584-586.[8]庄广伦.辅助生殖技术的发展历程[J].中国实用妇科与产科杂志,2010,26(10):729-731.[9]陈贵安.北京大学第三医院开展辅助生育技术近10年回顾[J].北京大学学报:医学版,2002,34(5):623-626.[10]张婷,王晓民.体外受精与试管婴儿—2010年诺贝尔生理学或医学奖简介[J].首都医科大学学报,2010,31(5):678-682.[11]乔杰,马彩虹.中国辅助生殖技术发展现状[J].国际生殖健康/计划生育杂志,2012,31(5):332-333.[12]王巍,马海兰.人类辅助生殖技术的应用现状[J].右江民族医学院学报,2011,33(3):353-355.[13]Quinn P. The development and impact of culture media for assisted reproductive technologies. Fertil Steril. 2004;81(1): 27-29.[14]Gardner DK, Vella P, Lane M, et al. Culture and transfer of human blastocysts increases implantation rates and reduces the need for multiple embryo transfers. Fertil Steril. 1998; 69(1):84-88.[15]赵杰,陈秀娟,范文斌.鼠胚在人类辅助生殖培养环境质量控制的作用研究[J].内蒙古医学院学报,2009,31(1):49-51.[16]Thompson JG, Bell AC, Tervit HR. Partitioning of glucose carbon in post-compaction ovine embryos. Anim Reprod Sci. 1995;38(1-2):119-126.[17]Biggers JD, Whittingham DG, Donahue RP. The pattern of energy metabolism in the mouse oöcyte and zygote. Proc Natl Acad Sci U S A. 1967;58(2):560-567.[18]Downs SM, Utecht AM. Metabolism of radiolabeled glucose by mouse oocytes and oocyte-cumulus cell complexes. Biol Reprod. 1999;60(6):1446-1452.[19]Eppig JJ, Hosoe M, O'Brien MJ, et al. Conditions that affect acquisition of developmental competence by mouse oocytes in vitro: FSH, insulin, glucose and ascorbic acid. Mol Cell Endocrinol. 2000;163(1-2):109-116.[20]Khurana NK, Niemann H. Effects of oocyte quality, oxygen tension, embryo density, cumulus cells and energy substrates on cleavage and morula/blastocyst formation of bovine embryos. Theriogenology. 2000;54(5):741-756.[21]Cetica P, Pintos L, Dalvit G, et al. Activity of key enzymes involved in glucose and triglyceride catabolism during bovine oocyte maturation in vitro. Reproduction. 2002;124(5): 675-681.[22]Steeves TE, Gardner DK. Metabolism of glucose, pyruvate, and glutamine during the maturation of oocytes derived from pre-pubertal and adult cows. Mol Reprod Dev. 1999;54(1): 92-101.[23]Gardner DK, Lane M, Calderon I, et al. Environment of the preimplantation human embryo in vivo: metabolite analysis of oviduct and uterine fluids and metabolism of cumulus cells. Fertil Steril. 1996;65(2):349-353. [24]吴正三.猪胚胎体外培养影响因素的研究[D].南宁:广西大学, 2006.[25]Houghton FD, Thompson JG, Kennedy CJ, et al. Oxygen consumption and energy metabolism of the early mouse embryo. Mol Reprod Dev. 1996;44(4):476-485. [26]Thompson JG, Partridge RJ, Houghton FD, et al. Oxygen uptake and carbohydrate metabolism by in vitro derived bovine embryos. J Reprod Fertil. 1996;106(2):299-306. [27]Martin KL, Leese HJ. Role of glucose in mouse preimplantation embryo development. Mol Reprod Dev. 1995;40(4):436-443. [28]Martin KL, Leese HJ. Role of developmental factors in the switch from pyruvate to glucose as the major exogenous energy substrate in the preimplantation mouse embryo. Reprod Fertil Dev. 1999;11(7-8):425-433. [29]Harris SE, Leese HJ, Gosden RG, et al. Pyruvate and oxygen consumption throughout the growth and development of murine oocytes. Mol Reprod Dev. 2009;76(3):231-238. [30]Gardner DK, Lane M, Batt P. Uptake and metabolism of pyruvate and glucose by individual sheep preattachment embryos developed in vivo. Mol Reprod Dev. 1993;36(3): 313-319. [31]Brown JJ, Whittingham DG. The dynamic provision of different energy substrates improves development of one-cell random-bred mouse embryos in vitro. J Reprod Fertil. 1992; 95(2):503-511. [32]Gardner DK, Clarke RN, Lechene CP, et al. Development of a noninvasive ultramicrofluorometric method for measuring net uptake of glutamine by single preimplantation mouse embryos. Gamete Res. 1989;24(4):427-438.[33]Downs SM, Houghton FD, Humpherson PG, et al. Substrate utilization and maturation of cumulus cell-enclosed mouse oocytes: evidence that pyruvate oxidation does not mediate meiotic induction. J Reprod Fertil. 1997;110(1):1-10.[34]吴嫦丽,张守全,高淑静,等.不同培养液对昆明小鼠体外受精率及早期胚胎体外发育的影响[J].华南农业大学学报, 2006,27(1): 107-109.[35]Leppens-Luisier G, Sakkas D. Development, glycolytic activity, and viability of preimplantation mouse embryos subjected to different periods of glucose starvation. Biol Reprod. 1997; 56(3):589-596.[36]邓彬,李予,王文军,等.胚胎培养液氨基酸浓度与体外受精-胚胎移植妊娠结局的关系[J].中山大学学报:医学科学版,2013, 34(6): 900-905.[37]王增艳,何方方,孙正怡,等.冷冻环玻璃化法冷冻小鼠卵母细胞的效果评价[J].生殖医学杂志,2008,17(1):38-42.[38]李宗武,朱传金,刘庆勇.人血浆及精浆中钙、镁、锌、铜对精液成分的影响[J].国外医学:医学地理分册,2004,25(3):124-126. |
.jpg)
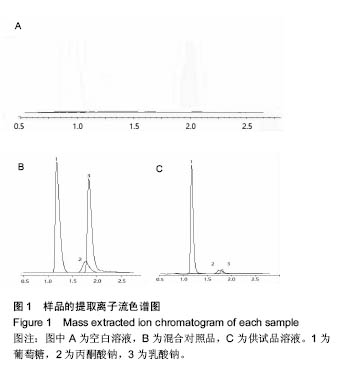
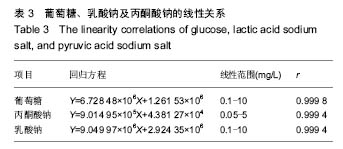
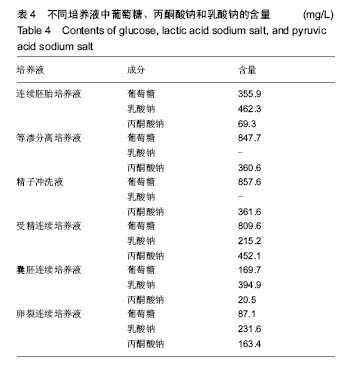
.jpg)
.jpg)
.jpg)