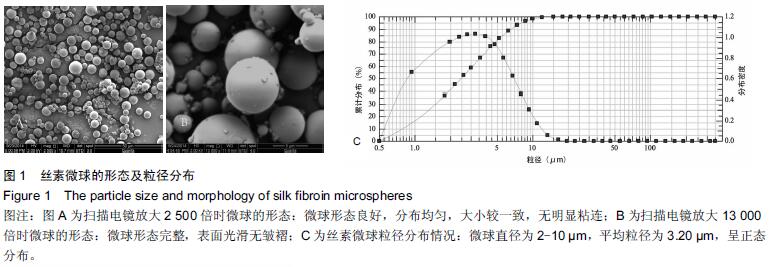
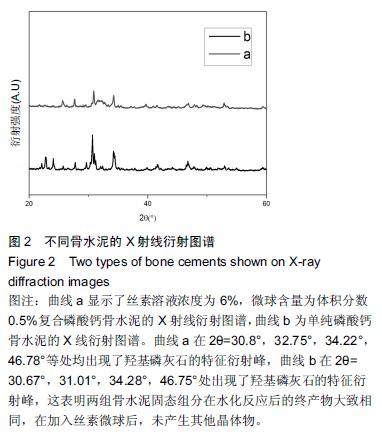
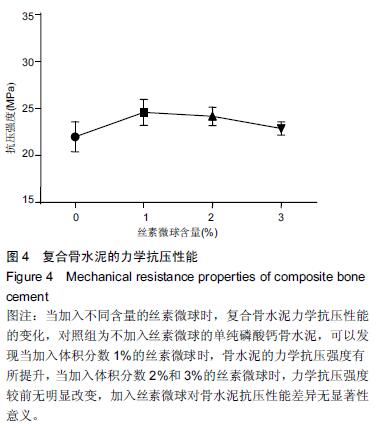
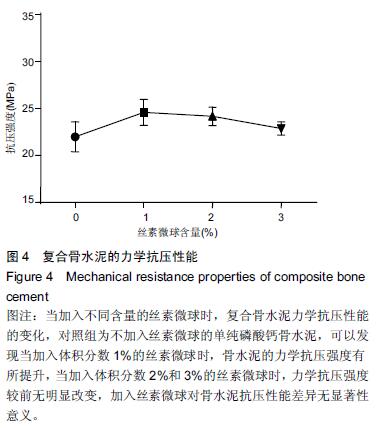
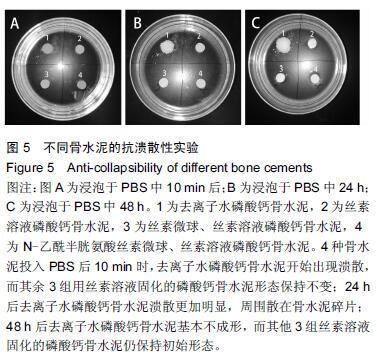
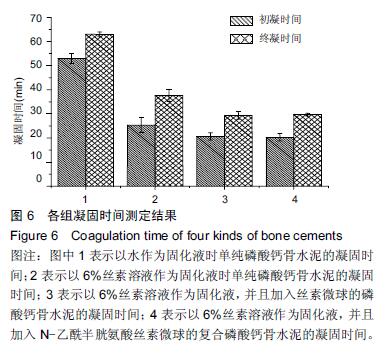
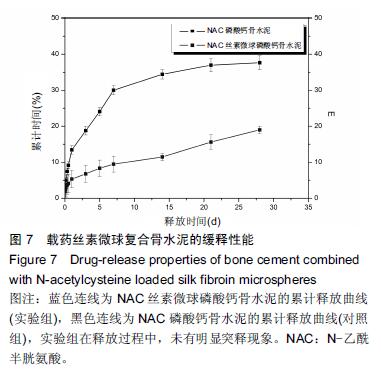
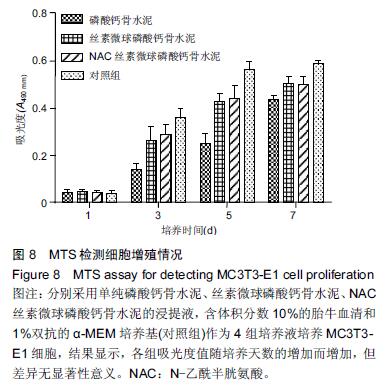
| [1] Nakamura T, Matsumine A, Asanuma K,et al. Treatment of bone defect with calcium phosphate cement subsequent to tumor curettage in pediatric patients. Oncol Lett. 2016;11(1):247-252. [2] Yuan H, Li Y, de Bruijn JD, et al. Tissue responses of calcium phosphate cement: a study in dogs. Biomaterials. 2000;21:1283-1290. [3] Ito T, Otsuka M. Application of calcium phosphate as a controlled-release device. Biol Pharm Bull. 2013; 36(11): 1676-1682. [4] Pastorino D, Canal C, Ginebra MP. Drug delivery from injectable calcium phosphate foams by tailoring the macroporosity-drug interaction. Acta Biomater. 2015: 250-259. [5] Kerckhofs G, Chai YC, Luyten FP. Combining microCT-based characterization with empirical modelling as a robust screening approach for the design of optimized CaP-containing scaffolds for progenitor cell-mediated bone formation. Acta Biomater. 2016. [6] Gu Y, Chen L, Yang HL, et al. Evaluation of an injectable silk fibroin enhanced calcium phosphate cement loaded with human recombinant bone morphogenetic protein-2 in ovine lumbar interbody fusion. J Biomed Mater ResA. 2011;97(2):177-185. [7] Chen L, Gu Y, Feng Y, et al. Bioactivity of porous biphasic calcium phosphate enhanced by recombinant human bone morphogenetic protein 2/silk fibroin microsphere. J Mater Sci Mater Med. 2014;25(7): 1709-1719. [8] Feng T, Pi B, Li B, et al. N-Acetyl cysteine (NAC)-mediated reinforcement of alpha-tricalcium phosphate/silk fibroin (α-TCP/SF) cement. Colloids Surf B Biointerfaces. 2015;136:892-899. [9] Nayak S, Dey S, Kundu SC. Silk sericin-alginate- chitosan microcapsules: hepatocytes encapsulation for enhanced cellular functions. Int J Biol Macromol. 2014; 65:258-266. [10] Wenk E, Merkle HP, Meinel L. Silk fibroin as a vehicle for drug delivery applications. J Control Release. 2011; 150: 128-141. [11] Tsukimura N, Yamada M, Aita H, et al. N-acetyl cysteine (NAC)-mediated detoxification and functionalization of poly (methyl methacrylate) bone cement. Biomaterials. 2009;30:3378-3389. [12] Yamada M, Tsukimura N, Ikeda T, et al. N-acetyl cysteine as an osteogenesis-enhanc ing molecule for bone regeneration. Biomaterials. 2013;34: 6147-6156. [13] Hofmann S, Wong CT, Rossetti F, et al. Silk fibroin as an organic polymer for controlled drug delivery. J Control Release. 2006;111:219-227. [14] Lu S, Wang P, Zhang F, et al. A novel silk fibroin nanofibrous membrane for guided bone regeneration: a study in rat calvarial defects. Am J Transl Res. 2015; 7(11):2244-2253. [15] Qiao C, Zhang K, Sun B, et al. Sustained release poly (lactic-co-glycolic acid) microspheres of bone morphogenetic protein 2 plasmid/calcium phosphate to promote in vitro bone formation and in vivo ectopic osteogenesis. Am J Transl Res. 2015;7(12):2561-2572. [16] Floren ML, Spilimbergo S, Motta A, et al. Carbon dioxide induced silk protein gelation for biomedical applications. Biomacromolecules. 2012;13(7):2060-2072. [17] Elia R, Guo J, Budijono S, et al. Encapsulation of Volatile Compounds in Silk Microparticles. J Coat Technol Res. 2015;12(4):793-799. [18] Ye M, Zeng S, Gao W, et al. Preparation and characterization of genipin-crosslinked silk fibroin/chitosan controlled-release microspheres. Nan Fang Yi Ke Da Xue Xue Bao. 2014;34(6):875-879. [19] Liu LS, Thompson AY, Heidaran MA, et al. An osteoconductive collagen/hyaluronate matrix for bone regeneration. Biomaterials. 1999;20(12): 1097-1108. [20] Budiraharjo R, Neoh KG, Kang ET. Hydroxyapatite-coated carboxymethyl chitosan scaffolds for promoting osteoblast and stem cell differentiation. J Colloid Interface Sci. 2012;366(1): 224-232. [21] 鲁红,田宇,吴织芬.珊瑚转化羟基磷灰石应用于牙周组织工程的细胞相容性和细胞毒性[J].中国组织工程研究, 2012,16(16):2955-2958. [22] Matsuoka H, Akiyama H, Okada Y, et al. In vitro analysis of the stimulation of bone formation by highly bioactive apatite- and wollastonite-containing glass-ceramic: released calcium ions promote osteogenic differentiation in osteoblastic ROS17/2.8 cells. J Biomed Mater Res. 1999;47(2):176-188. [23] Yang HL, Zhu XS, Chen L, et al. Bone healing response to a synthetic calcium sulfate/beta-tricalcium phosphate graft material in a sheep vertebral body defect model, J Biomed Mater Res B Appl Biomater. 2012;(100):1911-1921. [24] Zhang J, Liu W, Gauthier O, et al. A simple and effective approach to prepare injectable macroporous calcium phosphate cement for bone repair: syringe-foaming using a viscous hydrophilic polymeric solution. Acta Biomater. 2016;31:326-338. [25] Ratanavaraporn J, Kanokpanont S, Damrongsakkul S. The development of injectable gelatin/silk fibroin microspheres for the dual delivery of curcumin and piperine. J Mater Sci Mater Med. 2014;25(2):401-410. [26] Preda R, Leisk G, Omenetto F, et al. Bioengineered silk proteins to control cell and tissue functions. Methods Mol Biol. 2013;996:19-41. [27] Habraken WJ, Wolke JG, Mikos AG, et al. Porcine gelatin microsphere/calcium phosphate cement composites: an in vitro degradation study. J Biomed Mater Res B Appl Biomater. 2009;91(2):555-561. [28] 武莉,朱振锋,杨菁,等.影响微球药物释放因素的研究[J].生物医学工程与临床,2003,7(3):135-137. [29] 曹阳,王伯初,迟少萍,等.基于丝素蛋白的药物缓释材料[J].中国组织工程研究与临床康复,2009,13(8):1533-1536. [30] Link DP, van den Dolder J, Wolke JG, et al. The cytocompatibility and early osteogenic characteristics of an injectable calcium phosphate cement. Tissue Eng. 2007;13(3):493-500. [31] Feng T, Pi B, Li B, et al. N-Acetyl cysteine (NAC)-mediated reinforcement of alpha-tricalcium phosphate/silk fibroin (α-TCP/SF) cement. Colloids Surf B Biointerfaces. 2015;136:892-899. [32] Yu T, Jiang T, Wei QM, et al. Wound healing effects of silk fibroin-bone morphogenetic protein-2 scaffolds on inflammatory pulp in rats. Beijing Da Xue Xue Bao. 2015;47(5):814-819. [33] Buga MR, Zaharia C, B?lan M, et al. Surface modification of silk fibroin fibers with poly (methyl methacrylate) and poly (tributylsilyl methacrylate) via RAFT polymerization for marine antifouling applications. Mater Sci Eng C Mater Biol Appl. 2015; 51:233-241. [34] Gu Y, Chen L, Yang HL, et al. Evaluation of an injectable silk fibroin enhanced calcium phosphate cement loaded with human recombinant bone morphogenetic protein-2 in ovine lumbar interbody fusion. J Biomed Mater Res A. 2011;97(2):177-185. [35] Wang CH, Hsieh CY, Hwang JC. Flexible organic thin-film transistors with silk fibroin as the gate dielectric. Adv Mater. 2011;23(14):1630-1634. [36] Ding T, Yang H, Maltenfort M, et al. Silk fibroin added to calcium phosphate cement to prevent severe cardiovascular complications. Med Sci Monit. 2010; 16(9):HY23-HY26. [37] Qu Y, Yang Y, Li J, et al. Preliminary evaluation of a novel strong/osteoinductive calcium phosphate cement. J Biomater Appl. 2011;26(3):311-325. [38] Kim DH, Viventi J, Amsden JJ, et al. Dissolvable films of silk fibroin for ultrathin conformal bio-integrated electronics. Nat Mater. 2010;9(6):511-517. [39] Takeuchi A, Ohtsuki C, Miyazaki T, et al. Deposition of bone-like apatite on silk fiber in a solution that mimics extracellular fluid. J Biomed Mater Res A. 2003;65(2): 283-289. [40] Li YH, Wang ZD, Wang W, et al. The biocompatibility of calcium phosphate cements containing alendronate-loaded PLGA microparticles in vitro. Exp Biol Med (Maywood). 2015;240(11):1465-1471. [41] Simon CG Jr, Khatri CA, Wight SA, et al. Preliminary report on the biocompatibility of a moldable, resorbable, composite bone graft consisting of calcium phosphate cement and poly (lactide-co-glycolide) microspheres. J Orthop Res. 2002;20(3):473-482. |
.jpg)
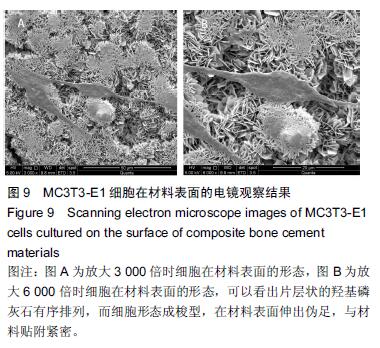
.jpg)