中国组织工程研究 ›› 2016, Vol. 20 ›› Issue (14): 2021-2026.doi: 10.3969/j.issn.2095-4344.2016.14.006
• 肿瘤干细胞 cancer stem cells • 上一篇 下一篇
胃癌干细胞在胃癌侵袭、转移中及血管形成中的作用和机制
黄 辉,潘志坚
- 华中科技大学同济医学院附属武汉中心医院,湖北省武汉市 430024
Role and mechanism of gastric cancer stem cells in gastric cancer invasion, metastasis and angiogenesis
Huang Hui, Pan Zhi-jian
- the Affiliated Wuhan Central Hospital of Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430024, Hubei Province, China
摘要:
文章快速阅读:
 文题释义:
肿瘤干细胞:首次在血液系统疾病中得到分离和鉴定,结果显示:多数实体肿瘤中均存在一类特殊的细胞,它们具备自我更新及自我分化能力,容易进一步促进肿瘤的发展。肿瘤干细胞在实体肿瘤,如:结肠癌、肝癌、胰腺癌中具备自我分化及更新能力,并且还具有高效DNA修复能力和多分化潜能。
血管生成:是指从已有的毛细血管或毛细血管后静脉发展而形成新的血管,主要包括:激活期血管基底膜降解;血管内皮细胞的激活、增殖、迁移;重建形成新的血管和血管网,是一个涉及多种细胞的多种分子的复杂过程。血管形成是促血管形成因子和抑制因子协调作用的复杂过程,正常情况下二者处于平衡状态,一旦此平衡打破就会激活血管系统,使血管生成过度或抑制血管系统使血管退化。
背景:肿瘤干细胞及其分化的血管细胞对于化疗、分子靶向治疗并不敏感,虽然能够减少肿瘤总体的血供,却助长了肿瘤的浸入及转移,成为治疗失败的主要机制。
目的:分析胃癌干细胞在胃癌侵袭、转移中的机制及其在血管形成中的作用。
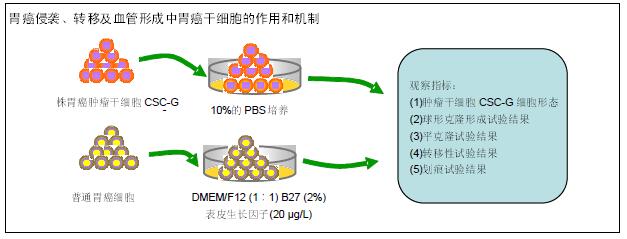
方法:取20株胃癌肿瘤干细胞CSC-G和普通胃癌细胞SGC7901细胞,普通癌细胞培养条件为10%的PBS;肿瘤干细胞CSC-G培养条件为:DMEM/F12(1∶1)、B27(2%)、表皮生长因子(20 μg/L)。对2种细胞进行球形克隆形成试验、平板克隆试验以及Transwell 侵袭性实验,观察胃癌肿瘤干细胞的侵袭性;测定CD44、E-cadherin中mRNA水平及蛋白表达水平,观察细胞的转移能力;利用细胞划痕试验、Transwell 迁移实验和成环实验观察细胞的血管形成能力。
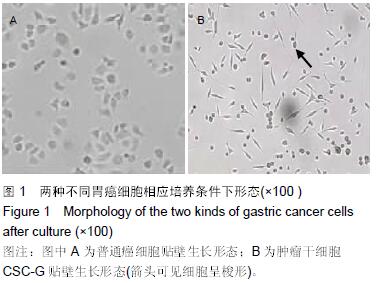
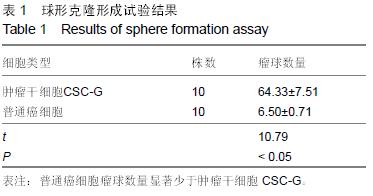
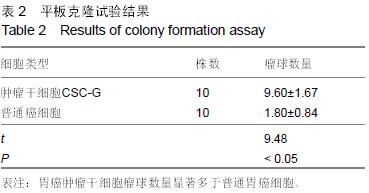
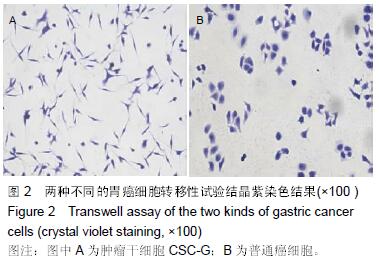
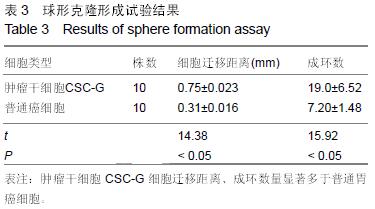
结果与结论:①普通癌细胞均贴壁生长,肿瘤干细胞CSC-G为悬浮生长,生长后显微镜下显示为梭形间质形态;②球形克隆形成试验结果:普通癌细胞瘤球数量显著少于肿瘤干细胞CSC-G(P < 0.05);③平克隆试验结果:胃癌肿瘤干细胞瘤球数量显著多余普通胃癌细胞(P < 0.05);④转移性试验结果:胃癌肿瘤干细胞穿过基底膜细胞数显著多于普通胃癌(P < 0.05);⑤划痕试验结果:肿瘤干细胞CSC-G迁移距离、成环试验中成环数量显著多于普通胃癌细胞(P < 0.05)。⑥结果提示:胃癌肿瘤干细胞侵袭能力高于普通胃癌细胞,且胃癌干细胞迁移能力以及血管成形能力较强,它可以通过形成血管等途径来进一步促进胃癌的发生与发展。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
ORCID: 0000-0003-0441-5887(黄辉)
文题释义:
肿瘤干细胞:首次在血液系统疾病中得到分离和鉴定,结果显示:多数实体肿瘤中均存在一类特殊的细胞,它们具备自我更新及自我分化能力,容易进一步促进肿瘤的发展。肿瘤干细胞在实体肿瘤,如:结肠癌、肝癌、胰腺癌中具备自我分化及更新能力,并且还具有高效DNA修复能力和多分化潜能。
血管生成:是指从已有的毛细血管或毛细血管后静脉发展而形成新的血管,主要包括:激活期血管基底膜降解;血管内皮细胞的激活、增殖、迁移;重建形成新的血管和血管网,是一个涉及多种细胞的多种分子的复杂过程。血管形成是促血管形成因子和抑制因子协调作用的复杂过程,正常情况下二者处于平衡状态,一旦此平衡打破就会激活血管系统,使血管生成过度或抑制血管系统使血管退化。
背景:肿瘤干细胞及其分化的血管细胞对于化疗、分子靶向治疗并不敏感,虽然能够减少肿瘤总体的血供,却助长了肿瘤的浸入及转移,成为治疗失败的主要机制。
目的:分析胃癌干细胞在胃癌侵袭、转移中的机制及其在血管形成中的作用。
方法:取20株胃癌肿瘤干细胞CSC-G和普通胃癌细胞SGC7901细胞,普通癌细胞培养条件为10%的PBS;肿瘤干细胞CSC-G培养条件为:DMEM/F12(1∶1)、B27(2%)、表皮生长因子(20 μg/L)。对2种细胞进行球形克隆形成试验、平板克隆试验以及Transwell 侵袭性实验,观察胃癌肿瘤干细胞的侵袭性;测定CD44、E-cadherin中mRNA水平及蛋白表达水平,观察细胞的转移能力;利用细胞划痕试验、Transwell 迁移实验和成环实验观察细胞的血管形成能力。
结果与结论:①普通癌细胞均贴壁生长,肿瘤干细胞CSC-G为悬浮生长,生长后显微镜下显示为梭形间质形态;②球形克隆形成试验结果:普通癌细胞瘤球数量显著少于肿瘤干细胞CSC-G(P < 0.05);③平克隆试验结果:胃癌肿瘤干细胞瘤球数量显著多余普通胃癌细胞(P < 0.05);④转移性试验结果:胃癌肿瘤干细胞穿过基底膜细胞数显著多于普通胃癌(P < 0.05);⑤划痕试验结果:肿瘤干细胞CSC-G迁移距离、成环试验中成环数量显著多于普通胃癌细胞(P < 0.05)。⑥结果提示:胃癌肿瘤干细胞侵袭能力高于普通胃癌细胞,且胃癌干细胞迁移能力以及血管成形能力较强,它可以通过形成血管等途径来进一步促进胃癌的发生与发展。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
ORCID: 0000-0003-0441-5887(黄辉)
.jpg)