中国组织工程研究 ›› 2015, Vol. 19 ›› Issue (18): 2928-2932.doi: 10.3969/j.issn.2095-4344.2015.18.025
• 器官移植动物模型 organ transplantation and animal model • 上一篇 下一篇
凋亡素原核表达载体构建及活性测定
张艳玲1,徐 霞2,江露含2,杜晶春2
- 1广州医科大学附属第五医院,广东省广州市 510700;2广州医科大学金域检验学院,广东省广州市 510182
Construction of a prokaryotic expression vector for apoptin and activity determination
Zhang Yan-ling1, Xu Xia2, Jiang Lu-han2, Du Jing-chun2
- 1The Fifth Affiliated Hospital of Guangzhou Medical University, Guangzhou 510700, Guangdong Province, China; 2Kingmed College of Laboratory Medicine, Guangzhou Medical University, Guangzhou 510182, Guangdong Province, China
摘要:
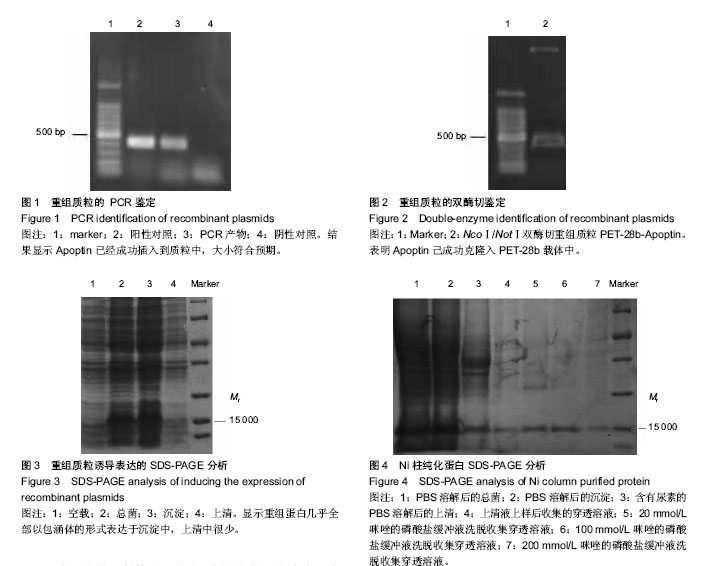
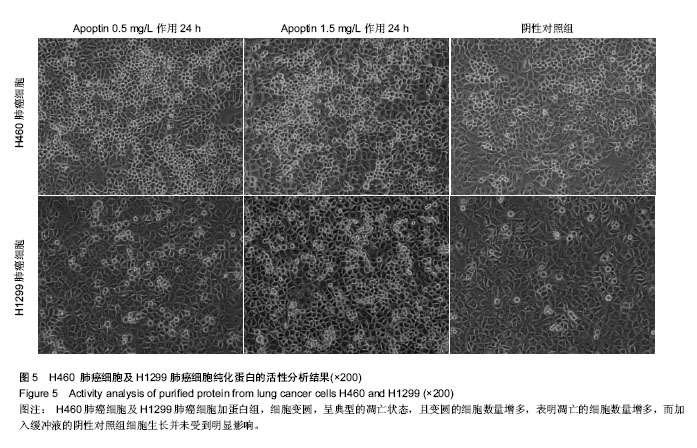
背景:体外合成或通过基因工程表达的凋亡素Apoptin 是一种可以特异性地诱导肿瘤细胞凋亡,而对人体正常细胞无毒性和转化活性的蛋白,为抑制肿瘤的生长提供可能。 目的:构建凋亡素基因的原核表达载体,优化诱导蛋白表达的相关条件,检测纯化所得目的蛋白的活性。 方法:将已构建好的凋亡素基因亚克隆至原核表达载体 pET-28b(+)中,将该质粒转化至大肠杆菌E.coli宿主菌中,以异丙基硫代半乳糖苷(IPTG)对其进行诱导表达,聚丙烯酰胺凝胶电泳(SDS-PAGE)分析目的蛋白,并检测所表达的目的蛋白对肿瘤细胞的增殖抑制作用。 结果与结论:凋亡素基因被成功的克隆至 pET-28b(+),在26 ℃、IPTG 0.5 mmol/L诱导8 h的情况下,Apoptin为包涵体表达,表达产物经 SDS-PAGE 分析,在相对分子质量约15 000的位置出现目的蛋白条带,大小与预期结果一致,经变复性和亲和层析纯化后,获得高纯度目的蛋白,进一步检测发现其对肺癌细胞H460及H1299具有一定的促凋亡作用。实验成功构建了凋亡素原核表达载体PET-28b-Apoptin,获得了具有一定生物活性的目的蛋白,为进一步研究凋亡素的功能和开发其应用价值研究奠定了基础。
中图分类号: