中国组织工程研究 ›› 2013, Vol. 17 ›› Issue (42): 7414-7419.doi: 10.3969/j.issn.2095-4344.2013.42.012
• 材料生物相容性 material biocompatibility • 上一篇 下一篇
磁性壳聚糖微球的生物相容性
王子妤1,董 菊1,张东生2
- 1南京中医药大学基础医学院,江苏省南京市 210023
2东南大学医学院,江苏省南京市 210009
Biocompatibility of magnetic chitosan microspheres
Wang Zi-yu1, Dong Ju1, Zhang Dong-sheng2
- 1School of Basic Medical Science, Nanjing University of Chinese Medicine, Nanjing 210023, Jiangsu Province, China
2School of Medical Science, Southeast University, Nanjing 210009, Jiangsu Province, China
摘要:
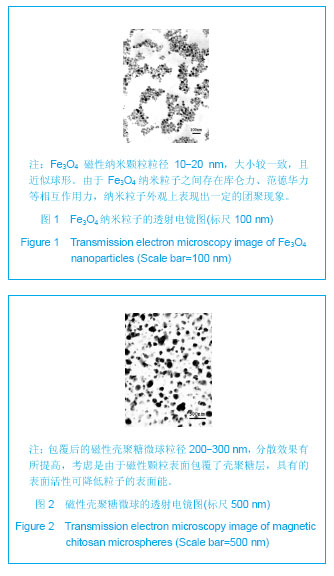
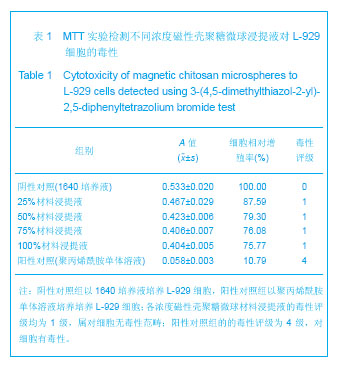
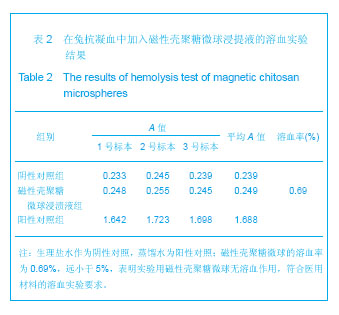
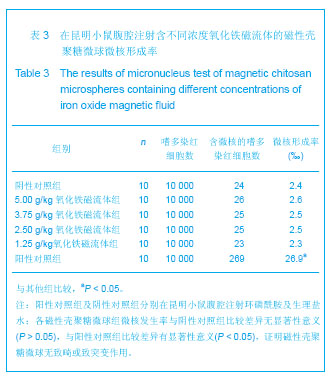
背景:采用壳聚糖对磁性氧化铁纳米颗粒表面进行改性,一方面可改善磁性纳米氧化铁颗粒的团聚性,增加其稳定性,另一方面将来可用于肿瘤热疗及基因治疗。 目的:制备将来可用于肿瘤热疗及基因治疗的磁性壳聚糖微球,评价其生物相容性。 方法:采用改良的化学沉淀法制备磁性氧化铁纳米颗粒。采用超声乳化法将壳聚糖加入到磁性氧化铁纳米颗粒中制备磁性壳聚糖微球,进行以下实验:①MTT实验检测磁性壳聚糖微球浸提液的细胞毒性:分别以1640培养液、聚丙烯酰胺单体溶液、100%,75%,50%,25%的磁性壳聚糖微球浸提液培养L-929细胞。②溶血实验:在兔抗凝血中分别加入磁性壳聚糖微球浸提液、生理盐水及蒸馏水。③微核实验:在昆明小鼠腹腔分别注射含氧化铁磁流体5,3.75,2.5,1.25 g/kg的磁性壳聚糖微球混悬液、环磷酰胺及生理盐水。 结果与结论:磁性壳聚糖微球粒径200-300 nm,分散效果有所提高。不同浓度的磁性壳聚糖微球浸提液对L-929 细胞毒性为1级,属对细胞无毒性范畴。磁性壳聚糖微球浸提液的溶血率为0.69%,小于5%,符合医用材料的溶血实验要求。磁性壳聚糖微球混悬液未导致细胞DNA断裂和非整倍体化,未导致微核产生的遗传毒理作用,材料无致畸或致突变作用,因此磁性壳聚糖微球具有良好的生物相容性。
中图分类号:
.jpg)
.jpg)
.jpg)
.jpg)