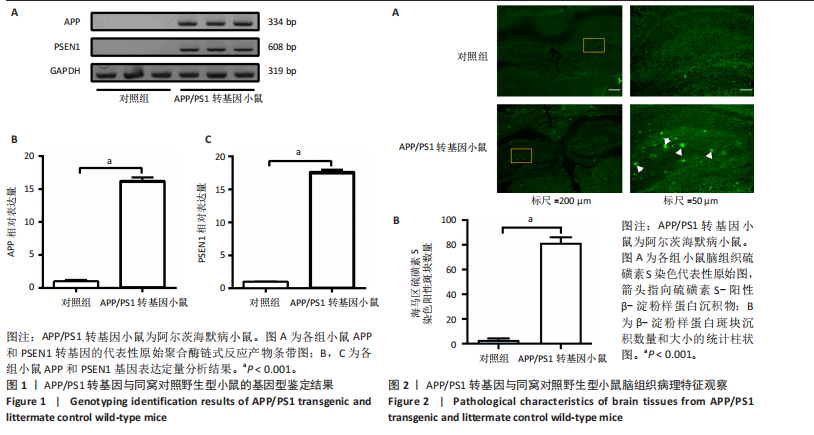
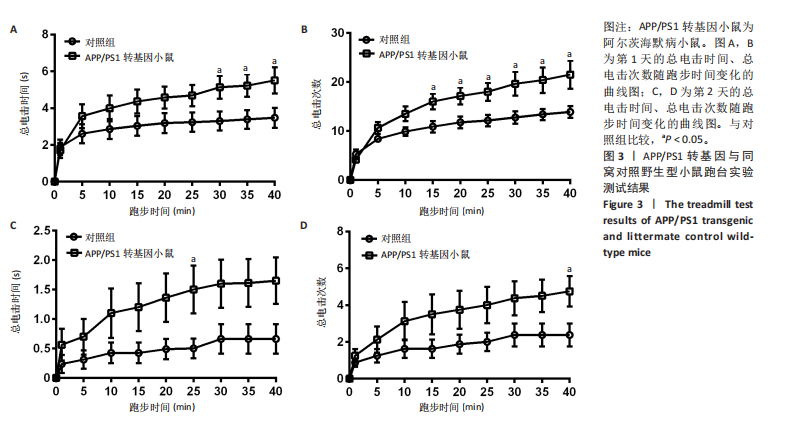
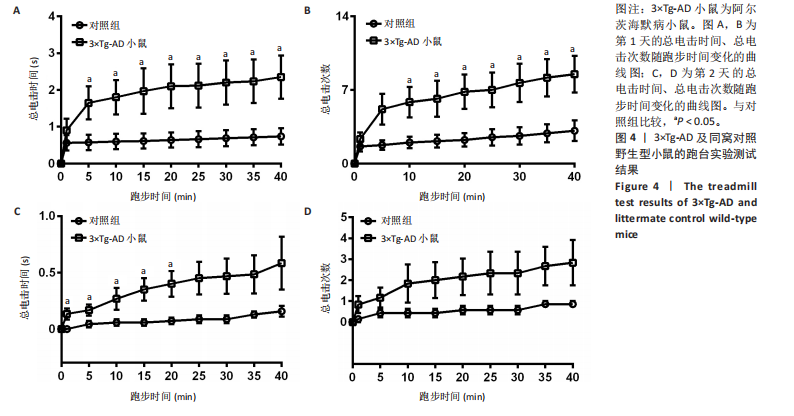
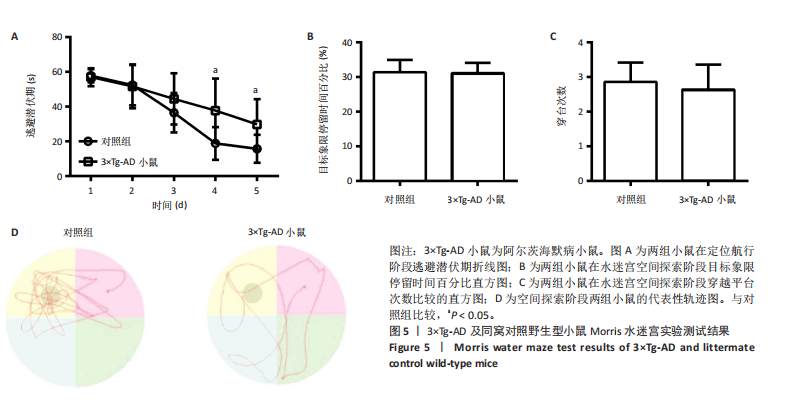
[1] ZHANG Y, CHEN H, LI R, et al. Amyloid β-based therapy for Alzheimer’s disease: challenges, successes and future. Signal Transduct Target Ther. 2023;8(1):248.
[2] OSSENKOPPELE R, VAN DER KANT R, HANSSON O. Tau biomarkers in Alzheimer’s disease: towards implementation in clinical practice and trials. Lancet Neurol. 2022;21(8):726-734.
[3] JANUS C, WESTAWAY D. Transgenic mouse models of Alzheimer’s disease. Physiol Behav. 2001;73(5):873-886.
[4] VORHEES CV, WILLIAMS MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1(2):848-858.
[5] ABDULAI-SAIKU S, GUPTA S, WANG D, et al. The maternal X chromosome affects cognition and brain ageing in female mice. Nature. 2025;638(8049):152-159.
[6] SHOJI H, TAKAO K, HATTORI S, et al. Contextual and cued fear conditioning test using a video analyzing system in mice. J Vis Exp. 2014;(85):50871.
[7] STOKES EG, VASQUEZ JJ, AZOUZ G, et al. Cationic peptides cause memory loss through endophilin-mediated endocytosis. Nature. 2025; 638(8050):479-489.
[8] SHI SM, SUH RJ, SHON DJ, et al. Glycocalyx dysregulation impairs blood-brain barrier in ageing and disease. Nature. 2025;639(8056):985-994.
[9] KRAEUTER AK, GUEST PC, SARNYAI Z. The Y-Maze for assessment of spatial working and reference memory in mice. Methods Mol Biol. 2019;1916:105-111.
[10] SCHROER AB, VENTURA PB, SUCHAROV J, et al. Platelet factors attenuate inflammation and rescue cognition in ageing. Nature. 2023; 620(7976):1071-1079.
[11] LI T, JIAO JJ, SU Q, et al. A GLP-1/GIP/Gcg receptor triagonist improves memory behavior, as well as synaptic transmission, neuronal excitability and Ca2+ homeostasis in 3xTg-AD mice. Neuropharmacology. 2020; 170:108042.
[12] HULSHOF LA, FRAJMUND LA, VAN NUIJS D, et al. Both male and female APPswe/PSEN1dE9 mice are impaired in spatial memory and cognitive flexibility at 9 months of age. Neurobiol Aging. 2022;113:28-38.
[13] DEACON RM, RAWLINS JN. T-maze alternation in the rodent. Nat Protoc. 2006;1(1):7-12.
[14] DOUGHERTY JP, SPRINGER DA, GERSHENGORN MC. The treadmill fatigue test: A simple, high-throughput assay of fatigue-like behavior for the mouse. J Vis Exp. 2016;(111):54052.
[15] REYNOLDS JC, LEE C. Mouse fitness as determined through treadmill running and walking. Methods Mol Biol. 2020;2144:57-65.
[16] WATTS CA, HAUPT A, SMITH J, et al. Measuring Skeletal Muscle Thermogenesis in Mice and Rats. J Vis Exp. 2022;(185):10.3791/64264.
[17] LI T, SU Q, ZHANG Z, et al. Ube2c-inhibition alleviated amyloid pathology and memory deficits in APP/PS1 mice model of AD. Prog Neurobiol. 2022;215:102298.
[18] KESIDOU E, THEOTOKIS P, DAMIANIDOU O, et al. CNS ageing in health and neurodegenerative disorders. J Clin Med. 2023;12(6):2255.
[19] CHESLOW L, SNOOK AE, WALDMAN SA. Biomarkers for managing neurodegenerative diseases. Biomolecules. 2024;14(4):398.
[20] GONZALES MM, GARBARINO VR, POLLET E, et al. Biological aging processes underlying cognitive decline and neurodegenerative disease. J Clin Invest. 2022;132(10):e158453.
[21] ALAFUZOFF I, LIBARD S. Ageing-related neurodegeneration and cognitive decline. Int J Mol Sci. 2024;25(7):4065.
[22] SCHELTENS P, DE STROOPER B, KIVIPELTO M, et al. Alzheimer’s disease. Lancet. 2021;397(10284):1577-1590.
[23] ZHANG J, ZHANG Y, WANG J, et al. Recent advances in Alzheimer’s disease: Mechanisms, clinical trials and new drug development strategies. Signal Transduct Target Ther. 2024;9(1):211.
[24] PÁDUA MS, GUIL-GUERRERO JL, PRATES JAM, et al. Insights on the use of transgenic mice models in Alzheimer’s disease research. Int J Mol Sci. 2024;25(5):2805.
[25] ZHONG MZ, PENG T, DUARTE ML, et al. Updates on mouse models of Alzheimer’s disease. Mol Neurodegener. 2024;19(1):23.
[26] KIM DH, JANG YS, JEON WK, et al. Assessment of cognitive phenotyping in inbred, genetically modified mice, and transgenic mouse models of Alzheimer’s disease. Exp Neurobiol. 2019;28(2):146-157.
[27] SANCHEZ-VARO R, MEJIAS-ORTEGA M, FERNANDEZ-VALENZUELA JJ, et al. Transgenic mouse models of Alzheimer’s disease: An integrative analysis. Int J Mol Sci. 2022;23(10):5404.
[28] TRINCHESE F, LIU S, BATTAGLIA F, et al. Progressive age-related development of Alzheimer-like pathology in APP/PS1 mice. Ann Neurol. 2004;55(6):801-814.
[29] ODDO S, CACCAMO A, SHEPHERD JD, et al. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39(3):409-421.
[30] YANG JT, WANG ZJ, CAI HY, et al. Sex differences in neuropathology and cognitive behavior in APP/PS1/tau triple-transgenic mouse model of Alzheimer’s disease. Neurosci Bull. 2018;34(5):736-746.
[31] 石辉,原丽,张军,等.APPswe/PS1dE9双转基因阿尔茨海默病模型小鼠的行为学及病理学特征研究[J]. 神经解剖学杂志,2016, 32(4):499-506.
[32] LI T, JIAO JJ, HÖLSCHER C, et al. A novel GLP-1/GIP/Gcg triagonist reduces cognitive deficits and pathology in the 3xTg mouse model of Alzheimer’s disease. Hippocampus. 2018;28(5):358-372.
[33] DETURE MA, DICKSON DW. The neuropathological diagnosis of Alzheimer’s disease. Mol Neurodegener. 2019;14(1):32.
[34] AKHTAR A, SINGH S, KAUSHIK R, et al. Types of memory, dementia, Alzheimer’s disease, and their various pathological cascades as targets for potential pharmacological drugs. Ageing Res Rev. 2024;96:102289.
[35] SU Q, LI T, HE PF, et al. Trichostatin A ameliorates Alzheimer’s disease-related pathology and cognitive deficits by increasing albumin expression and Aβ clearance in APP/PS1 mice. Alzheimers Res Ther. 2021;13(1):7.
|