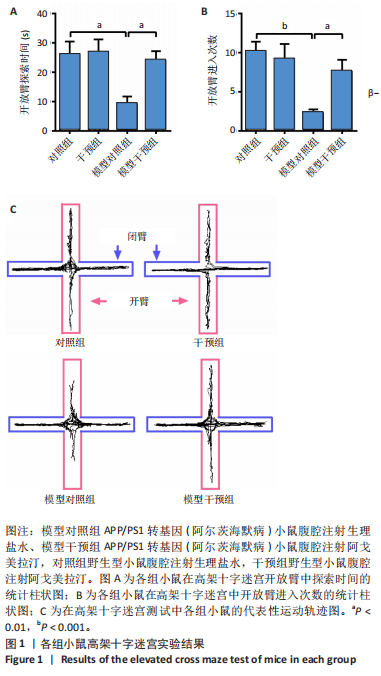
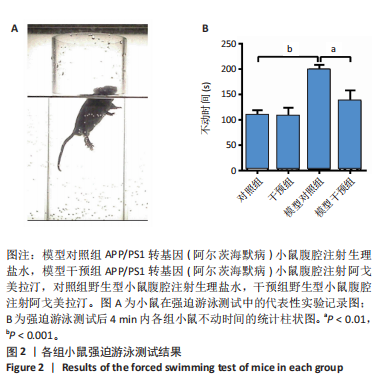
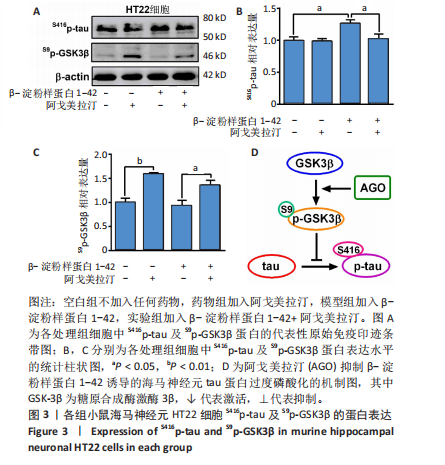
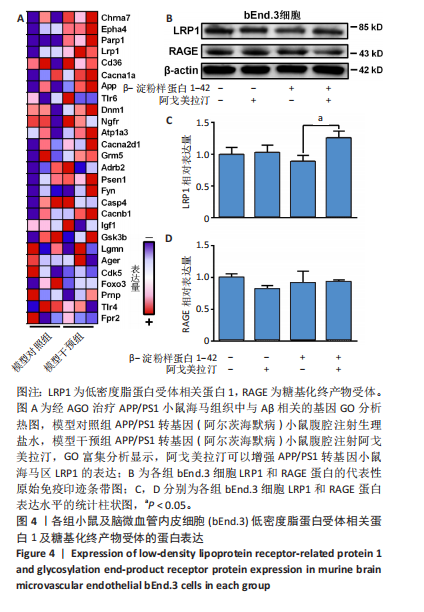
[1] CACABELOS R. What have we learnt from past failures in Alzheimer’s disease drug discovery? Expert Opin Drug Discov. 2022;17(4):309-323.
[2] BUSCHE MA, HYMAN BT. Synergy between amyloid-β and tau in Alzheimer’s disease. Nat Neurosci. 2020;23(10):1183-1193.
[3] CHEN Y, DANG M, ZHANG Z. Brain mechanisms underlying neuropsychiatric symptoms in Alzheimer’s disease: a systematic review of symptom-general and -specific lesion patterns. Mol Neurodegener. 2021;16(1):38.
[4] GRACIA-GARCIA P, BUENO-NOTIVOL J, LIPNICKI DM, et al. Clinically significant anxiety as a risk factor for Alzheimer’s disease: Results from a 10-year follow-up community study. Int J Methods Psychiatr Res. 2023;32(3):e1934.
[5] BOTTO R, CALLAI N, CERMELLI A, et al. Anxiety and depression in Alzheimer’s disease: a systematic review of pathogenetic mechanisms and relation to cognitive decline. Neurol Sci. 2022;43(7):4107-4124.
[6] HASAN SMN, CLARKE C, STRAND T, et al. Putative pathological mechanisms of late-life depression and Alzheimer’s disease. Brain Res. 2023;1813:148423.
[7] PATEL P, MASURKAR AV. The relationship of anxiety with Alzheimer’s disease: a narrative review. Curr Alzheimer Res. 2021;18(5):359-371.
[8] CACABELOS R, CARRIL JC, CORZO L, et al. Pharmacogenetics of anxiety and depression in Alzheimer’s disease. Pharmacogenomics. 2023;24(1):27-57.
[9] SU Q, LI T, LIU GW, et al. Agomelatine: a potential novel approach for the treatment of memory disorder in neurodegenerative disease. Neural Regen Res. 2023;18(4):727-733.
[10] LI T, SU Q, ZHANG Z, et al. Ube2c-inhibition alleviated amyloid pathology and memory deficits in APP/PS1 mice model of AD. Prog Neurobiol. 2022;215:102298.
[11] KRAEUTER AK, GUEST PC, SARNYAI Z. The elevated plus maze test for measuring anxiety-Like behavior in rodents. Methods Mol Biol. 2019; 1916:69-74.
[12] YANKELEVITCH-YAHAV R, FRANKO M, HULY A, et al. The forced swim test as a model of depressive-like behavior. J Vis Exp. 2015(97):52587.
[13] KRELL-ROESCH J, RAKUSA M, SYRJANEN JA, et al. Association between CSF biomarkers of Alzheimer’s disease and neuropsychiatric symptoms: Mayo Clinic Study of Aging. Alzheimers Dement. 2023;19(10):4498-4506.
[14] LEYDER E, SURESH P, JUN R, et al. Depression-related phenotypes at early stages of Aβ and tau accumulation in inducible Alzheimer’s disease mouse model: Task-oriented and concept-driven interpretations. Behav Brain Res. 2023;438:114187.
[15] HUANG Z, LIN HWK, ZHANG Q, et al. Targeting Alzheimer’s disease: the critical crosstalk between the liver and brain. Nutrients. 2022; 14(20):4298.
[16] RAUCH JN, LUNA G, GUZMAN E, et al. LRP1 is a master regulator of tau uptake and spread. Nature. 2020;580(7803):381-385.
[17] DEANE R, DU YAN S, SUBMAMARYAN RK, et al. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat Med. 2003;9(7):907-913.
[18] PASSERI E, ELKHOURY K, MORSINK M, et al. Alzheimer’s disease: treatment strategies and their limitations. Int J Mol Sci. 2022;23(22):13954.
[19] LIVINGSTON G, HUNTLEY J, SOMMERLAD A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413-446.
[20] JIA L, DU Y, CHU L, et al. Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: a cross-sectional study. Lancet Public Health. 2020; 5(12):e661-e671.
[21] ASMER MS, KIRKHAM J, NEWTON H, et al. Meta-analysis of the prevalence of major depressive disorder among older adults with dementia. J Clin Psychiatry. 2018;79(5):17r11772.
[22] CHEN P, GUARINO PD, DYSKEN MW, et al. Neuropsychiatric symptoms and caregiver burden in individuals with Alzheimer’s disease: the TEAM-AD VA cooperative study. J Geriatr Psychiatry Neurol. 2018;31(4): 177-185.
[23] LEUNG DKY, CHAN WC, SPECTOR A, et al. Prevalence of depression, anxiety, and apathy symptoms across dementia stages: A systematic review and meta-analysis. Int J Geriatr Psychiatry. 2021;36(9):1330-1344.
[24] JOURDAN JP, BUREAU R, ROCHAIS C, et al. Drug repositioning: a brief overview. J Pharm Pharmacol. 2020;72(9):1145-1151.
[25] SAVINO R, POLITO AN, MARSALA G, et al. Agomelatine: a potential multitarget compound for neurodevelopmental disorders. Brain Sci. 2023;13(5):734.
[26] MILLAN MJ. Agomelatine for the treatment of generalized anxiety disorder: focus on its distinctive mechanism of action. Ther Adv Psychopharmacol. 2022;12:20451253221105128.
[27] YU Y, CHEN Y, MA L, et al. Efficacy of agomelatine with cognitive behavioral therapy for delayed sleep-wake phase disorder in young adults: A randomized controlled study. Behav Sleep Med. 2023;21(5): 529-539.
[28] CHEN Y, LI J, LIAO M, et al. Efficacy and safety of agomelatine versus SSRIs/SNRIs for post-stroke depression: a systematic review and meta-analysis of randomized controlled trials. Int Clin Psychopharmacol. 2024;39(3):163-173.
[29] ARMSTRONG SM, MCNULTY OM, GUARDIOLA-LEMAITRE B, et al. Successful use of S20098 and melatonin in an animal model of delayed sleep-phase syndrome (DSPS). Pharmacol Biochem Behav. 1993;46(1):45-49.
[30] YOUS S, ANDRIEUX J, HOWELL HE, et al. Novel naphthalenic ligands with high affinity for the melatonin receptor. J Med Chem. 1992;35(8):1484-1486.
[31] MILLAN MJ, GOBERT A, LEJEUNE F, et al. The novel melatonin agonist agomelatine (S20098) is an antagonist at 5-hydroxytryptamine2C receptors, blockade of which enhances the activity of frontocortical dopaminergic and adrenergic pathways. J Pharmacol Exp Ther. 2003; 306(3):954-964.
[32] STAHL SM. Mechanism of action of agomelatine: a novel antidepressant exploiting synergy between monoaminergic and melatonergic properties. CNS Spectr. 2014;19(3):207-212.
[33] MENDEZ MF. The relationship between anxiety and Alzheimer’s disease. J Alzheimers Dis Rep. 2021;5(1):171-177.
[34] DAFSARI FS, JESSEN F. Depression-an underrecognized target for prevention of dementia in Alzheimer’s disease. Transl Psychiatry. 2020;10(1):160.
[35] SAIZ-VAZQUEZ O, GRACIA-GARCIA P, UBILLOS-LANDA S, et al. Depression as a risk factor for Alzheimer’s disease: a systematic review of longitudinal meta-analyses. J Clin Med. 2021;10(9):1809.
[36] BABULAL GM, ROE CM, STOUT SH, et al. Depression is associated with tau and not amyloid positron emission tomography in cognitively normal adults. J Alzheimers Dis. 2020;74(4):1045-1055.
[37] EGASHIRA N, IWASAKI K, TAKASHIMA A, et al. Altered depression-related behavior and neurochemical changes in serotonergic neurons in mutant R406W human tau transgenic mice. Brain Res. 2005;1059(1):7-12.
|