中国组织工程研究 ›› 2026, Vol. 30 ›› Issue (16): 4166-4179.doi: 10.12307/2026.351
• 组织构建综述 tissue construction review • 上一篇 下一篇
铁死亡抑制剂治疗骨关节炎:多样性和多靶点特征
陈鑫龙1,2,孟 涛1,王耀敏1,2,张克凡1,李 健1,石 辉1,张晨晨1
- 1滨州医学院附属医院,山东省滨州市 256600;2滨州医学院,山东省滨州市 264003
-
收稿日期:2025-05-15接受日期:2025-08-18出版日期:2026-06-08发布日期:2025-11-28 -
通讯作者:石辉,博士,主任医师,滨州医学院附属医院,山东省滨州市 256600 共同通讯作者:张晨晨,滨州医学院附属医院,山东省滨州市 256600 -
作者简介:陈鑫龙,男,2000年生,山东省滨州市人,汉族,滨州医学院在读硕士,主要从事骨关节与运动医学研究。 共同第一作者:孟涛,男,1973年生,山东省滨州市人,汉族,副主任医师,主要从事骨关节与运动医学研究。 -
基金资助:山东省省级临床重点专科学科建设项目(SLCZDZK-0302),项目负责人:石辉;山东省医药卫生科技项目(202304070630),项目负责人:李健
Ferroptosis inhibitors in the treatment of osteoarthritis: diversity and multitarget characteristics
Chen Xinlong1, 2, Meng Tao1, Wang Yaomin1, 2, Zhang Kefan1, Li Jian1, Shi Hui1, Zhang Chenchen1
- 1Affiliated Hospital of Binzhou Medical University, Binzhou 256600, Shandong Province, China; 2Binzhou Medical University, Binzhou 264003, Shandong Province, China
-
Received:2025-05-15Accepted:2025-08-18Online:2026-06-08Published:2025-11-28 -
Contact:Shi Hui, PhD, Chief physician, Affiliated Hospital of Binzhou Medical University, Binzhou 256600, Shandong Province, China Co-corresponding author: Zhang Chenchen, Affiliated Hospital of Binzhou Medical University, Binzhou 256600, Shandong Province, China -
About author:Chen Xinlong, MS candidate, Affiliated Hospital of Binzhou Medical University, Binzhou 256600, Shandong Province, China; Binzhou Medical University, Binzhou 264003, Shandong Province, China Meng Tao, Associate chief physician, Affiliated Hospital of Binzhou Medical University, Binzhou 256600, Shandong Province, China Chen Xinlong and Meng Tao contributed equally to this work. -
Supported by:Shandong Provincial Clinical Key Specialty Construction Project, No. SLCZDZK-0302 (to SH); Shandong Medical and Health Science and Technology Project, No. 202304070630 (to LJ)
摘要:
文题释义:
铁死亡:是一种铁依赖性的细胞死亡方式,由细胞内脂质过氧化物的异常积累引发,在形态学、生物化学和遗传学上区别于凋亡、坏死和自噬等其他细胞死亡方式。
活性氧:是指氧在代谢过程中产生的一系列具有高反应活性的分子和自由基,主要包括超氧化物阴离子(O₂⁻)、过氧化氢(H₂O₂)、羟基自由基(·OH)、单线态氧(¹O₂)。
背景:研究表明,铁死亡作为一种新型的铁依赖性细胞死亡方式,在干预骨关节炎发展进程中发挥重要作用。
目的:介绍铁死亡的机制,包括铁稳态失衡、脂质过氧化和抗氧化体系减弱,并总结了多种铁死亡抑制剂在骨关节炎治疗中的应用前景。
方法:第一作者于2024年1月应用计算机在中国知网和PubMed数据库检索2012年1月至2025年1月发表的相关文献,以“骨关节炎,铁死亡,脂质过氧化,铁死亡抑制剂,软骨细胞,活性氧,谷胱甘肽过氧化物酶4”为中文检索词,以“osteoarthritis,ferroptosis,lipid peroxidation,ferroptosis inhibitors,chondrocytes,reactive oxygen species,glutathione peroxidase 4”为英文检索词,对最终纳入的90篇文献进行了系统性的总结和归纳。
结果与结论:①铁死亡是一种铁依赖性的细胞死亡方式,其核心机制如下:铁稳态失衡,细胞内过量的铁经芬顿反应产生活性氧,致使脂质过氧化以及细胞死亡;脂质过氧化,活性氧侵袭细胞膜中的多不饱和脂肪酸,造成细胞膜降解并引发铁死亡;抗氧化体系减弱,细胞内的抗氧化系统(如Xc-系统/谷胱甘肽/谷胱甘肽过氧化物酶4、核因子E2相关因子2、丝裂原活化蛋白激酶/核因子κB等信号通路)在铁死亡过程中发挥关键作用,当抗氧化能力无法应对脂质过氧化时,细胞便会发生铁死亡。②针对铁死亡在骨关节炎中的作用,多种铁死亡抑制剂呈现出治疗潜力,铁螯合剂凭借螯合过量的铁,减少芬顿反应以及脂质过氧化,抑制软骨细胞铁死亡;抗氧化剂借助抑制脂质过氧化以及提高抗氧化能力,减轻软骨细胞损伤;天然化合物通过调节核因子E2相关因子2、丝裂原活化蛋白激酶/核因子κB等信号通路,抑制铁死亡并缓解骨关节炎进展;此外,酰基辅酶A合成酶长链家族成员4抑制剂通过抑制脂质过氧化和纠正铁代谢紊乱,发挥软骨保护作用。③虽然铁死亡抑制剂在骨关节炎治疗方面呈现出广阔前景,不过当前多数研究仍处于细胞和动物实验阶段,缺少大规模临床试验来验证其安全性与有效性。未来研究应剖析铁死亡的具体机制,并推动铁死亡抑制剂的临床应用,为骨关节炎治疗提供新策略。
https://orcid.org/0009-0008-6764-8692(陈鑫龙);https://orcid.org/0009-0003-0730-4461(孟涛);https://orcid.org/0000-0002-0515-2746(石辉);https://orcid.org/0009-0000-8557-0624(张晨晨)
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
中图分类号:
引用本文
陈鑫龙, 孟 涛, 王耀敏, 张克凡, 李 健, 石 辉, 张晨晨. 铁死亡抑制剂治疗骨关节炎:多样性和多靶点特征[J]. 中国组织工程研究, 2026, 30(16): 4166-4179.
Chen Xinlong, Meng Tao, Wang Yaomin, Zhang Kefan, Li Jian, Shi Hui, Zhang Chenchen. Ferroptosis inhibitors in the treatment of osteoarthritis: diversity and multitarget characteristics[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(16): 4166-4179.
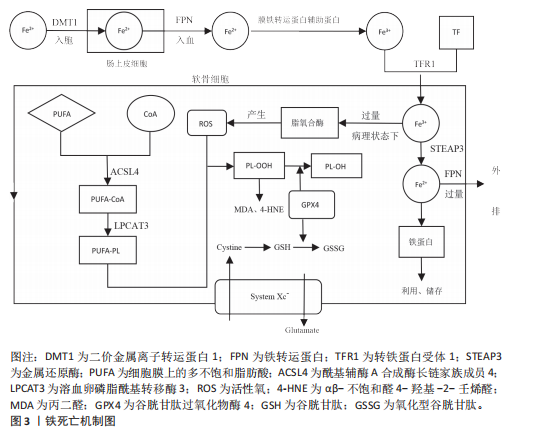
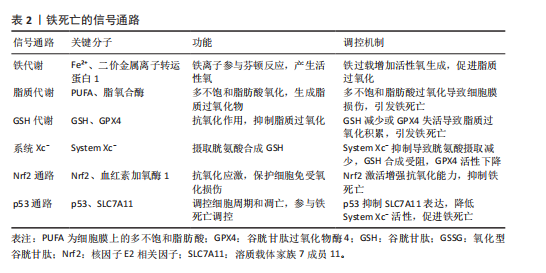
2.2 铁死亡在骨关节炎中的调节机制 铁死亡与骨关节炎的发展密切相关,在软骨细胞铁死亡的整个过程中,铁稳态的失衡及脂质过氧化被认为是核心步骤,随后脂质过氧化物的形成及抗氧化体系减弱直接导致铁死亡。
2.2.1 铁稳态的失衡 铁是人体最重要的基本元素之一,参与人体各种各样的生命活动。人体摄入的铁(主要是Fe2+)在十二指肠及空肠上段中通过二价金属离子转运蛋白1转运到肠上皮细胞中[11],然后通过铁转运蛋白入血,随后被膜铁转运蛋白辅助蛋白转换为Fe3+后被运送至身体各器官发挥作用。进入血液循环中的Fe3+首先与
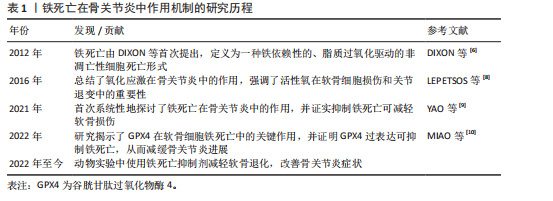
转铁蛋白结合,将Fe3+输送到外周组织。与转铁蛋白结合的Fe3+在转铁蛋白与细胞膜上的转铁蛋白受体1结合后,通过受体介导的内吞作用被细胞摄取。在细胞内,Fe3+被金属还原酶还原为Fe2+,而后Fe2+通过二价金属离子转运蛋白1进入细胞质,并以铁蛋白的形式利用或储存[12]。当Fe2+含量过多时,一部分Fe2+还可以通过铁转运蛋白实现外排作用。在病理状态下,当细胞内铁的含量过多时,Fe2+可通过芬顿反应和激活含铁酶(如脂氧合酶)导致活性氧的产生,而活性氧的产生和随后的脂质过氧化与软骨细胞的抗氧化能力有关,在软骨降解和软骨细胞铁死亡中起关键作用[13]。
2.2.2 脂质过氧化 脂质过氧化可以被描述为氧化剂(如活性氧)攻击含有碳碳双键的脂质的过程,磷脂是细胞膜的重要组成部分,而磷脂的结构中含有大量多不饱和脂肪酸(polyunsaturated fatty acids,PUFA),首先PUFAs在酰基辅酶 A 合成酶长链家族成员4(acyl-coA synthetase long-chain family member 4,ACSL4)的催化下与CoA结合合成PUFA-CoA,随后经溶血卵磷脂酰基转移酶3的作用下将PUFA-CoA插入膜磷脂(phospholipid,PL)中以形成PUFA-PL[14],活性氧则是通过攻击PUFA-PL来启动软骨细胞脂质过氧化,进而细胞膜发生降解而导致铁死亡。脂质过氧化的阶段包括起始、传播和终止,在起始阶段,活性氧与PUFA-PL反应并提取烯丙基氢生成脂质自由基;在传播阶段,脂质自由基与氧反应形成脂质过氧自由基,然后脂质过氧自由基与PUFA反应生成新的自由基和脂质氢过氧化物。在终止阶段,抗氧化剂或脂质自由基可以通过向脂质过氧自由基提供氢原子来阻止传播,从而形成非自由基产物[15]。由于脂质氢过氧化物的不稳定性,它们可能会降解成次级产物,αβ-不饱和醛4-羟基-2-壬烯醛(4-Hydroxy-2-Nonenal,4-HNE)和丙二醛是主要的次级产物,也是铁死亡关键的生物学标志物[16],而4-HNE和丙二醛具有细胞毒性,可与软骨细胞DNA碱基、蛋白质和其他亲核分子反应,导致软骨细胞死亡。尽管4-HNE和丙二醛与铁死亡密切相关,在铁死亡中发挥着核心作用,但它们也存在于其他细胞死亡方式中,如细胞凋亡过程中,在某些情况下可能伴随脂质过氧化,导致丙二醛和4-HNE生成。
2.2.3 抗氧化体系的减弱 当脂质过氧化物的产生压倒细胞本身的抗氧化缓冲能力时便会发生铁死亡,细胞本身有多种抗氧化体系来控制铁死亡,以System Xc-/谷胱甘肽(glutathione,GSH)/谷胱甘肽过氧化物酶4(glutathione peroxidase 4,GPX4)途径是细胞触发铁死亡的关键途径[17],Xc-即胱氨酸谷氨酸逆向转运蛋白,由2个关键亚基[溶质载体家族7成员11(solute carrier family 7 member 11,SLC7A11)和溶质载体家族3成员2]组成,主要作用是将细胞内的谷氨酸以1∶1的比例交换细胞外的胱氨酸,胱氨酸进入细胞内紧接着被还原为半胱氨酸用于合成GSH,GPX4则是一种含硒代半胱氨酸且GSH依赖性的酶,其可以使用GSH作为辅助因子来解毒细胞脂质过氧化过程中形成的脂质氢过氧化物[18]。因此System Xc-介导的胱氨酸摄取或GSH耗竭导致GPX4失活,允许脂质过氧化物积累,从而触发铁死亡。此外,已有研究发现GPX4下调通过丝裂原活化蛋白激酶(mitogen-activated protein kinase, MAPK)/核因子κB(nuclear factor kappa-B,NF-κB)信号通路激活软骨分解代谢相关蛋白,促进细胞外基质降解[10]。核因子E2相关因子2(nuclear factor-erythroid 2-related factor-2, Nrf2)是一种细胞抗氧化分子。在正常情况下,Nrf2与其抑制蛋白Keap1结合,保持在细胞质中并被泛素化降解。当细胞受到氧化应激时,Nrf2与Keap1解离,进入细胞核,结合抗氧化反应元件,激活多种抗氧化基因的表达。Nrf2的激活对于减轻软骨细胞脂质过氧化和铁死亡至关重要,激活Nrf2信号通路可增强骨关节炎中软骨细胞对氧化应激的抵御能力,抑制细胞凋亡,降低炎症因子的表达[19]。Nrf2的激活可通过抑制MAPK磷酸化,间接抑制NF-κB炎症通路,从而减轻氧化应激和铁死亡[20]。肿瘤抑制因子p53则可以通过抑制SLC7A11的转录,降低Xc-活性而减少胱氨酸的摄取和GSH的合成,导致细胞抗氧化能力下降,进而引起铁死亡发生[21]。p53和Nrf2在调控铁死亡中表现出拮抗作用。p53的激活可以抑制Nrf2的活性,从而增强铁死亡;而Nrf2的激活则可能抵消p53的促铁死亡作用[22]。
铁死亡机制原理图见图3,铁死亡的信号通路见表2。
2.3 铁死亡抑制剂在治疗骨关节炎的作用
2.3.1 铁螯合剂
(1)去铁胺(Deferoxamine):去铁胺是美国食品药品监督管理局批准的一种有效的铁螯合剂,用于抑制各种退行性疾病模型中的铁死亡。去铁胺具有较强的亲铁性,可以特异性与Fe3+离子形成稳定的络合物并促进其排出体外,因此去铁胺常用于铁过载类疾病[23]。关节软骨由蛋白聚糖和Ⅱ型胶原蛋白组成,而基质金属蛋白酶被认为是主要的基质降解酶,参与骨关节炎中细胞外基质的分解[24]。在骨
关节炎的治疗中,已有多项实验表明,去铁胺可以抑制白细胞介素1β刺激的软骨细胞中基质金属蛋白酶的表达。GUO等[25]运用叔丁基过氧化氢诱导软骨细胞及运用不稳定内侧半月板手术建立体内及体外骨关节炎模型评估去铁胺干预骨关节炎进展实验中发现,过量的铁可诱导软骨细胞衰老,而去铁胺可以通过螯合过量的铁,来恢复铁蛋白水平,减缓软骨细胞衰老;在体内实验中,对小鼠关节内注射去铁胺增强了骨关节炎小鼠软骨Ⅱ型胶原蛋白的表达,延缓了软骨退化。而在另一项实验中,GUO等[26]应用白细胞介素1β来模拟炎症,应用内侧半月板失稳小鼠模型在体内模拟骨关节炎,并将erastin注射到关节腔中以诱导小鼠膝关节软骨细胞铁死亡。研究结果表明,去铁胺减弱了基质金属蛋白酶表达的上调,并抑制白细胞介素1β诱导的Ⅱ型胶原蛋白表达的下调;研究数据还显示,去铁胺抑制了软骨细胞中活性氧的积累,进一步抑制了白细胞介素1β刺激的丙二醛和Fe2+的产生。去铁胺还显著改善了软骨细胞铁死亡导致的细胞内线粒体损伤,经去铁胺处理后的软骨细胞内线粒体膜更完整,线粒体更窄,嵴数量更多。此外,去铁胺通过促进Nrf2的表达和易位来激活软骨细胞中的Nrf2抗氧化系统,进而增强软骨细胞抗氧化能力。MIAO等[10]也通过观察过氧化叔丁醇诱导的骨关节炎体外细胞模型和前交叉韧带横断面诱导的骨关节炎小鼠体内模型发现,去铁胺有效减少了细胞内丙二醛水平的增加且逆转了GSH过氧化物酶活性、GSH含量和GSH/氧化型谷胱甘肽(L-Glutathione Oxidized,GSSG)的下降,因此得出结论:去铁胺可以通过抑制铁死亡来挽救过氧化叔丁醇诱导的细胞死亡,减少脂质过氧化、减少铁积累和维持GPX4功能。以上结果表明,去铁胺可以通过抑制软骨细胞铁死亡来调节白细胞介素1β诱导的软骨细胞炎症反应,达到治疗骨关节炎的作用。尽管去铁胺于细胞以及动物研究里呈现出一定疗效,然而在治疗人体骨关节炎时存在诸多局限,去铁胺有可能引发贫血等不良反应,在长期使用的情况下表现得更为突出,而且在非铁过载状态时,其铁螯合效应或许会对正常铁代谢造成干扰,当前有关去铁胺治疗骨关节炎的研究多数处于基础实验阶段,缺少大规模、多中心以及长期的临床研究数据作为支撑。对于去铁胺在骨关节炎治疗中的疗效与安全性,需要更多大规模临床研究来加以验证,在未来研究中,可以在临床运用纳米载体技术开发基于聚乳酸-羟基乙酸共聚物或者环糊精聚合物的纳米颗粒[27-28],这样可提高关节局部药物浓度并减少全身暴露,降低全身不良反应,另外还应研发联合治疗策略,把去铁胺和其他抗氧化剂联合起来以达到治疗骨关节炎更佳的效果。
(2)姜黄素:姜黄素是一种天然的化合物,是姜黄根茎中的主要单体成分,具有多种潜在的生物活性。目前,姜黄素已被用于阿育吠陀和传统中医以治疗关节炎,可以显著改善关节疼痛和僵硬[29]。姜黄素具有一定的铁螯合能力,姜黄素分子结构中的β-二酮结构可以与铁离子发生配位作用,从而形成稳定的螯合物[30]。但姜黄素作为铁螯合剂的作用相对较弱,与传统的铁螯合剂(如去铁胺)等相比,其螯合能力和效果有一定的差距。不过,姜黄素除了铁螯合作用外,还具有抗炎、抗氧化等多种生物活性。既往实验证明,姜黄素对骨关节炎也具有一定的治疗作用[31]。ZHOU等[32]利用erastin构建骨关节炎铁死亡细胞模型和小鼠膝关节炎模型,并使用姜黄素干预发现,姜黄素显著降低了软骨细胞中铁死亡相关指标,如Fe2+、活性氧及丙二醛的积累,并上调了抗铁死亡蛋白GPX4、SLC7A11的表达,下调了促铁死亡蛋白ACSL4和转铁蛋白受体1的表达;此外,干扰Nrf2的基因和蛋白质表达水平显著削弱了姜黄素对软骨细胞的保护作用,并减弱了软骨细胞对氧化应激的抵抗力。因此,该研究得出结论,姜黄素通过激活Nrf2通路在体外和体内预防骨关节炎。陈凡等[33]发现姜黄素可上调铁死亡过程中过氧化物酶6的蛋白表达,促进过氧化物酶6清除活性氧,降低脂质过氧化的生物标记物水平且抑制铁过载,从而抑制erastin诱导的软骨细胞铁死亡。而在小规模的临床试验中也证实,姜黄素通过抑制炎症因子和抗氧化作用改善骨关节炎症状[34]。综上所述,多种实验结果表明,姜黄素作为铁死亡抑制剂在治疗骨关节炎中起到了一定的作用,但姜黄素水溶性差、代谢快,导致口服后生物利用度极低,虽然纳米载体或联合给药(如与硫酸软骨素)可部分改善这一问题[35-36],但如何实现高效递送仍是临床应用的重大挑战。
2.3.2 抗氧化剂
(1)铁抑素1(Ferrostatin-1):铁抑素1是一种有效的、选择性的铁死亡抑制剂,主要通过抑制细胞内脂质过氧化来发挥作用,特异性地抑制铁死亡的发生[6]。YAO等[9]使用白细胞介素1β干预软骨细胞来模拟炎症,使用柠檬酸铁铵来模拟体外铁过载,研究发现铁抑素1可以部分减轻白细胞介素1β和柠檬酸铁铵诱导的细胞毒性,降低细胞内脂质活性氧水平,铁抑素1也能够提升GPX4和SLC7A11的表达,抑制P53的表达,增强软骨细胞抗氧化能力,起到抑制软骨细胞铁死亡的作用;另外运用不稳定内侧半月板手术模拟小鼠体内骨关节炎模型发现,关节内注射铁抑素1显著减轻了小鼠软骨降解并促进Ⅱ型胶原蛋白的表达,延缓了小鼠骨关节炎的进展。WANG等[37]通过构建白细胞介素1β诱导软骨细胞铁死亡体外模型,以及内侧半月板失稳小鼠体内骨关节炎模型发现,铁抑素1不仅可以减轻白细胞介素1β诱导的软骨细胞炎症反应和细胞外基质降解,调节p53/SLC7A11/GPX4信号通路改善软骨细胞铁死亡,还可以维持铁死亡过程中的线粒体膜电位来减轻线粒体损伤,并且可以降低细胞质内铁和线粒体内铁水平,改善线粒体超微结构的损伤。XU等[38]在全膝关节置换术患者废弃的软骨中提取软骨细胞,并使用白细胞介素1β进行刺激,模拟骨关节炎的炎症环境,研究结果表明,铁抑素1逆转了白细胞介素1β刺激导致的软骨细胞形态改变、活力下降和增殖减少,且减少了细胞内Fe3?积累和氧化应激标志物的变化。上述实验所得到的结果显示,铁抑素1可通过抑制软骨细胞铁死亡发挥缓解骨关节炎的功效,然而铁抑素1在体内容易被迅速代谢,致使生物利用度较低,抑制铁死亡的效价不够充足,鉴于这一局限性,ZHANG等[39]运用化学修饰合成了新型铁抑素1类似物,使血浆稳定性得到明显提升,在体外实验里呈现出比铁抑素1更强的抑制铁死亡的能力。目前的研究大多集中在铁抑素1借助抑制铁死亡来缓解骨关节炎,可是骨关节炎的病理机制较为复杂,涉及炎症、氧化应激等多个通路,单一靶点或许疗效有限,未来的研究需要探寻与其他药物联合使用时产生的协同效应,提高铁抑素1的疗效,也可研究纳米递送系统,提升药物局部浓度,减少全身不良反应。总之铁抑素1有望突破当前的局限,成为骨关节炎治疗的潜在选择。
(2) XJB-5-131:XJB-5-131是一种线粒体靶向的抗氧化剂和自由基清除剂,具有电子清除和抗氧化的作用。KRAINZ等[40]提出,XJB-5-131可以通过抑制线粒体内脂质过氧化来抑制细胞铁死亡,对铁死亡相关疾病具有治疗意义。在XJB-5-131治疗骨关节炎的研究中,SUN等[41]通过构建叔丁基过氧化氢诱导的小鼠软骨细胞铁死亡体外模型和小鼠内侧半月板失稳骨关节炎体内模型发现,XJB-5-131在体外可以缓解叔丁基过氧化氢诱导的软骨细胞活力下降,减轻软骨细胞内活性氧、铁离子积累等铁死亡标志物,发挥抗软骨细胞铁死亡的作用。在体内,关节内注射XJB-5-131可以减轻骨关节炎小鼠模型的骨赘形成和软骨下骨硬化,可促进软骨细胞合成代谢蛋白以及铁死亡抑制因子的表达,并且能降低软骨细胞基质金属蛋白酶以及铁死亡驱动因子的表达,从而抑制软骨细胞铁死亡。Pebp1是一种多功能蛋白,在铁死亡调控里发挥着关键作用,它可抑制软骨细胞的铁死亡以及炎症反应,保护软骨细胞,延缓骨关节炎的发展。SUN等[41]经RNA基因测序发现,XJB-5-131借助恢复Pebp1表达抑制软骨细胞铁死亡并减轻骨关节炎的发展。因此,XJB-5-131为干预骨关节炎的发展提供了治疗思路,不过目前对XJB-5-131的研究还处在细胞及动物实验阶段,临床前数据有限,给药方式以及长期用药可能存在的不良反应还未评估。未来研究应着重于临床试验以及靶向递送系统开发,推动XJB-5-131成为有潜力的骨关节炎治疗药物。
(3)利普司他汀1(Liproxstatin-1):利普司他汀1是一种铁死亡抑制剂,主要通过抑制脂质过氧化物来发挥作用,从而减少铁死亡的发生。据以往研究报道,利普司他汀1可以通过增加GPX4水平来减轻铁过载,同时降低丙二醛和活性氧的水平来抑制细胞铁死亡[42]。在CHENG等[43]的研究中探讨了利普司他汀1在颞下颌关节骨关节炎中的治疗作用,发现利普司他汀1可以抑制白细胞介素1β诱导的下颌髁突软骨细胞铁死亡,减少活性氧、铁离子和丙二醛的积累,维持线粒体的形态和功能,改善软骨细胞外基质成分表达。XU等[38]也对比了铁抑素1和利普司他汀1对软骨细胞的作用,发现铁抑素1和利普司他汀1都能减轻骨关节炎软骨细胞铁死亡,但铁抑素1在保护软骨细胞免受骨关节炎炎症环境影响方面效果更好。以上研究结果表明,利普司他汀1可能成为骨关节炎治疗的潜在药物。在临床应用中,利普司他汀1也存在着药物递送、疗效证据不足、临床转化等方面的不足,未来研究应注重多靶点联合干预、递送技术革新及临床规模试验方面,以推动其成为骨关节炎治疗的疾病修饰药物。
(4) α-酮戊二酸:α-酮戊二酸是三羧酸循环中的关键中间体,是生物体极为重要的物质,在细胞中具有抗氧化、抗炎和胶原蛋白合成等关键作用。ETV转录因子是ETS基因家族的一个亚家族,它在基因表达调控中起重要作用,参与细胞增殖、分化、凋亡和肿瘤发生等过程。ETV4 通过与启动子结合来增强SLC7A11转录,进而增强GPX4发挥解毒作用,防止细胞脂质过氧化。HE等[44]构建了大鼠内侧半月板失稳骨关节炎体内模型,并运用H?O?诱导软骨细胞氧化应激体外模型发现,α-酮戊二酸显著抑制了H?O?诱导的软骨细胞基质降解和凋亡,降低活性氧和丙二醛水平。此外,经基因测序和蛋白质测序发现,α-酮戊二酸在mRNA和蛋白水平上上调ETV4、SLC7A11和GPX4的表达,减少Fe2?的积累,保护线粒体膜电位,从而抑制铁死亡,从而得出结论,α-酮戊二酸可以通过ETV4/SLC7A11/GPX4通路阻断铁死亡来缓解骨关节炎进展。但α-酮戊二酸的半衰期较短,且细胞膜穿透能力有限,导致局部有效浓度难以维持。为应对这一局限性,WANG等[45]开发自组装的纳米颗粒结合了透明质酸和α-酮戊二酸的优点,提高了α-酮戊二酸在膝关节内的稳定性和靶向性。
(5)褪黑激素(Melatonin):褪黑激素是一种由人体内松果体腺分泌的胺类激素,其主要功能是调节睡眠周期。大量研究表明,褪黑激素具有抗氧化、抗炎、抗肿瘤和抗衰老的特性,可以清除体内氧自由基,刺激内源性抗氧化酶,并提高其他抗氧化剂的效率[46]。NADPH氧化酶4是NADPH氧化酶家族的一员,大量存在于软骨细胞线粒体中,可诱导线粒体能量代谢紊乱和功能障碍,从而加剧氧化应激和脂质过氧化[47],因此,NADPH氧化酶4过表达会导致软骨细胞内活性氧、丙二醛水平显著增高,在调节骨关节炎铁死亡过程中具有重要作用。WANG等[48]运用白细胞介素1β诱导软骨细胞损伤和氧化应激的骨关节炎体外模型,以及采用前交叉韧带切断术建立小鼠骨关节炎体内模型发现,褪黑激素通过抑制NADPH氧化酶4的表达,减轻线粒体功能障碍和铁死亡,从而缓解骨关节炎。在临床试验中,口服褪黑激素展示出缓解膝骨关节炎患者疼痛的良好潜力[49]。但褪黑激素口服给药易被肝脏氧化降解,且缺乏关节组织特异性靶向能力,LIANG等[50]设计了一种纳米颗粒可持续释放褪黑激素并靶向软骨,关节内注射这种纳米颗粒可以减少注射次数并提高褪黑激素的体内可用性。褪黑激素治疗骨关节炎的潜力受限于递送效率、机制复杂性和个体差异,但通过纳米技术、联合治疗及机制深化研究,有望突破这些瓶颈,未来需更多临床试验验证其长期安全性和疗效。
(6)米托蒽醌(Mitoquinone):米托蒽醌是一种线粒体靶向抗氧化剂,在减轻线粒体氧化损伤方面比非靶向抗氧化剂更为有效,因此,米托蒽醌对各种氧化损伤相关疾病具有保护功能。Parkin是一种在人体中具有重要生物学功能的蛋白质,在PINK1-Parkin通路中发挥重要作用,具有清除受损或功能失调的线粒体的作用[51]。HOU等[52]运用白细胞介素1β诱导软骨细胞骨关节炎体外模型,并构建内侧半月板失稳小鼠创伤性骨关节炎体内模型发现,米托蒽醌能够激活Nrf2通路,减少细胞外基质的降解和炎症反应;米托蒽醌还增加了GPX4的表达,降低了活性氧的水平,抑制了软骨细胞的铁死亡。此外,该研究还发现,Parkin通过负反馈调节Nrf2的表达,敲低Parkin会增加Nrf2的表达。因此,米托蒽醌通过激活Nrf2信号通路和线粒体自噬,抑制铁死亡和炎症反应,减轻了骨关节炎的进展。但在临床应用转化中,米托蒽醌还有许多局限性,如靶向性与递送效率较低、对人体的不良反应尚未评估等,还需要开展进一步的临床研究。
(7)卡泊三醇(Calcipotriol):卡泊三醇是一种维生素D衍生物,现已被研究为银屑病的治疗剂。已有研究发现,卡泊三醇在骨关节炎患者培养的软骨细胞中发挥持久的抗炎和抗增殖作用[53]。转化生长因子β1是一种极其重要的细胞因子,在软骨细胞中具有双重作用,对于维持关节软骨、代谢稳态和结构完整性至关重要,而转化生长因子β1水平异常可能导致基质金属蛋白酶和炎症递质的过度表达,加速软骨退化[54]。经YANG等[55]运用白细胞介素1β诱导软骨细胞骨关节炎体外模型,并构建内侧半月板失稳小鼠骨关节炎体内模型发现,卡泊三醇显著降低了白细胞介素1β诱导的活性氧和脂质过氧化水平,并增加了GPX4的表达,且通过抑制转化生长因子β1来抑制白细胞介素1β诱导的软骨细胞炎症反应,进一步抑制了GPX4介导的铁死亡。联合使用卡泊三醇和转化生长因子β1特异性抑制剂或许能提升对骨关节炎的治疗成效,然而高剂量维生素D或者其类似物可能会给软骨和骨骼带来不良作用,动物实验说明,虽说低剂量的卡泊三醇在关节炎模型里未发现软骨或骨组织有明显损害,不过长期高剂量使用的安全性仍要验证,故而在临床应用时要依据患者维生素D代谢状况个体化调整剂量,防止高剂量毒性。另外还需要开展多中心随机对照试验,评估卡泊三醇在人类骨关节炎中的长期安全性以及疾病修饰作用。
2.3.3 ACSL4抑制剂
(1)罗格列酮:罗格列酮是一种常用的治疗糖尿病的药物。已有研究表明,罗格列酮可以抑制ACSL4活性来阻碍PFUA-PL的产生,从而抑制肾小管上皮细胞和肠上皮细胞中的脂质过氧化和铁死亡[56-57]。在CAO等[58]的研究中发现,经罗格列酮干预过的软骨细胞炎症及铁死亡模型中软骨细胞及其线粒体内铁离子显著降低,纠正了铁代谢紊乱;同时,软骨细胞及其线粒体内的活性氧水平显著降低,恢复了GSH和GSSG的平衡。此外,经过多种检测方法发现,罗格列酮可以减轻白细胞介素1β诱导的线粒体、溶酶体、内质网及高尔基体等膜性细胞器的损伤,改善线粒体膜电位。在体内实验中,运用内侧半月板失稳手术和右旋糖酐铁给药建立了小鼠骨关节炎模型并在关节腔内注射罗格列酮,结果发现关节腔内注射罗格列酮可减轻关节软骨退变、炎症反应,表明罗格列酮在体内具有保护作用。因此,CAO等[58]的研究结果表明,罗格列酮通过抑制脂质过氧化和纠正铁代谢紊乱发挥软骨保护和抗铁死亡的作用。但罗格那酮作为胰岛素增敏剂,可能引起体质量增加、血脂异常和胰岛素抵抗,这对合并代谢综合征的骨关节炎患者不利。此外,罗格那酮治疗骨关节炎的临床试验还不足,还需设计多中心随机对照试验,评估罗格那酮对骨关节炎患者疼痛、关节功能及影像学进展的影响。
(2)丹皮酚(Paeonol):丹皮酚作为一种小分子酚类化合物,有较强的抗炎、抗氧化以及抗血小板能力。已有相关研究指出,丹皮酚可以通过抑制ACSL4的活性来防止神经元中的脂质过氧化和铁死亡[59]。CAO等[60]运用白细胞介素1β和柠檬酸铁铵诱导的软骨细胞铁死亡体内实验,以及内侧半月板失稳手术体外实验,来研究丹皮酚对于骨关节炎所起的作用。体外实验结果显示,丹皮酚凭借靶向抑制ACSL4活性,明显减少了白细胞介素1β和柠檬酸铁铵诱导的软骨细胞炎症损伤以及铁死亡现象,同时减轻了软骨细胞线粒体损伤;在小鼠体内骨关节炎模型中,向关节腔内注射丹皮酚可减轻软骨退变,抑制骨赘形成。上述实验结果说明,丹皮酚依靠抑制ACSL4可有效抑制骨关节炎进展过程中软骨细胞的降解以及铁死亡。BIHLET等[61]评估了丹皮酚和儿茶酚固定剂量组合对有症状的膝骨关节炎患者的影响,发现丹皮酚和儿茶酚固定剂量组合在治疗时并未明显改善骨关节炎患者的症状,不过在特定亚组中呈现出潜在疗效,未来需要开展大规模临床试验研究,以明确丹皮酚对骨关节炎的疗效与安全性,并且剖析丹皮酚与其他药物联用的协同治疗机制。
2.3.4 其他天然化合物抑制剂 据大量研究发现,多种天然化合物可以通过抑制软骨细胞铁死亡达到治疗骨关节炎的作用。
小豆蔻素是一种从生姜中提取的天然化合物,具有抗炎和抗氧化作用。GONG等[62]使用白细胞介素1β刺激大鼠软骨细胞以模拟骨关节炎的炎症环境,以及通过大鼠内侧半月板失稳手术诱导骨关节炎体内模型,研究表明,小豆蔻素显著减轻了白细胞介素1β诱导的炎症反应和软骨基质降解,增加了Ⅱ型胶原蛋白、p53、SLC7A11和GPX4的表达。此外,关节内注射小豆蔻素显著改善了软骨损伤,表明小豆蔻素可以通过p53/SLC7A11/GPX4信号通路减轻软骨细胞铁死亡。Sirtuins是参与各种细胞功能的蛋白质家族,沉默信息调节因子2相关酶1(silent mating type information regulation 2 homolog-1,SIRT1)是Sirtuin家族的一员,是一种烟酰胺腺嘌呤二核苷酸依赖性组蛋白脱乙酰酶,具有抗炎、抗氧化和抗凋亡作用[63]。LI等[64]通过腹腔内注射铁葡聚糖建立铁过载小鼠模型,并结合内侧半月板失稳手术诱导膝关节骨关节炎体内模型;使用柠檬酸铁铵处理软骨细胞,模拟铁过载环境建立体外模型。研究发现,软骨细胞铁过载可以抑制SIRT1的表达,从而加强软骨细胞内炎症反应,小豆蔻素则可以通过激活SIRT1抑制MAPK通路和活性氧的产生,从而抑制软骨细胞炎症反应和软骨细胞铁死亡。据以上实验结果表明,小豆蔻素可以通过抑制软骨细胞铁死亡而达到治疗骨关节炎的作用。但其临床应用仍存在以下局限性:小豆蔻素作为天然化合物,存在水溶性低、口服吸收差的问题,且缺乏大规模的临床试验验证其有效性与安全性,因此未来需着重解决生物利用度与安全性问题,并设计针对性临床试验验证其疗效。
生物素A是一种具有抗炎、抗氧化、抗肿瘤等多种药理作用的天然化合物。HE等[65]用糊精铁腹腔注射到小鼠体内,建立铁超负荷小鼠模型并通过手术诱导骨关节炎体内模型,另外运用柠檬酸铁铵诱导软骨细胞建立骨关节炎体外模型实验发现,生物素A可以直接减轻膝关节内铁的沉积及炎症反应,并降低软骨细胞内细胞内铁浓度,抑制转铁蛋白受体1,促进铁转运蛋白,同时靶向激活Nrf2/system Xc-/GPX4信号通路,清除活性氧,防止脂质过氧化,抑制软骨细胞铁死亡,缓解骨关节炎的进程。目前生物素A治疗骨关节炎的临床前或临床试验较少,缺乏疗效和安全性数据支持,未来需进一步设计严谨的临床试验,评估生物素A单独或联合使用方案对骨关节炎患者症状的改善情况。
虾青素是一种酮式类胡萝卜素,因其独特的分子结构而具有强大的抗氧化能力。 WANG等[66]采用白细胞介素1β诱导大鼠软骨细胞构建骨关节炎体外模型,以及运用大鼠内侧半月板失稳手术诱导大鼠骨关节炎体内模型,研究虾青素在治疗骨关节炎方面的作用。研究结果显示,虾青素可有效减轻白细胞介素1β诱导产生的软骨细胞炎症反应以及细胞外基质降解,借助调节P53/SLC7A11/GPX4信号通路抑制铁死亡,保护软骨细胞线粒体结构,改善线粒体功能,发挥缓解骨关节炎的功效。在临床试验中,STONEHOUSE等[67]评估了富含虾青素的磷虾油对轻度至中度膝骨关节炎成年患者膝关节疼痛、僵硬以及身体功能的改善情况,研究得出,磷虾油安全且对膝骨关节炎症状有一定程度改善,特别适用于炎症水平较高的患者。由于虾青素在人体内的生物利用率较低且具有细胞毒性,有学者研发了一种含有虾青素的纳米脂质体制剂,用以提高其生物利用度并降低细胞毒性,保护骨骼免受氧化应激与炎症影响。
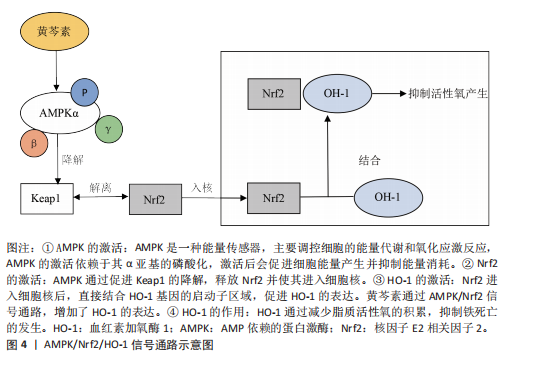
黄芩素是一种从黄芩中提取的类黄酮,具有抗炎和抗氧化等特性。WAN等[68]通过小鼠内侧半月板失稳体内实验,以及对白细胞介素1β诱导骨关节炎微环境中的软骨细胞观察发现,关节内注射黄芩素显著减轻了小鼠软骨退化、骨赘形成以及软骨下骨硬化状况,并降低骨关节炎相关的疼痛敏感性。黄芩素通过促进AMP依赖的蛋白激酶(AMP-activated protein kinase,AMPK)α亚基发生磷酸化,提高了AMPK全酶的组装与稳定性,激活Nrf2并推动其进入细胞核。Nrf2进入细胞核后,依靠上调血红素加氧酶1(heme oxygenase-1,HO-1)的表达,抑制了软骨细胞的脂质过氧化,从而抑制了铁死亡,见图4。因此,黄芩素可通过提升AMPK/Nrf2/HO-1信号传导的活性来抑制软骨细胞铁死亡,从而延缓骨关节炎的发展进程。目前对于黄芩素缓解骨关节炎进展的研究大多基于短期动物实验,长期毒性以及剂量依赖性效应有待依靠临床试验进行评估。
苦柯胺(Kukoamine)是一种在枸杞、番茄等植物中提取出的天然化合物,具有抗炎、抗肿瘤等多种生物活性。SUN等[69]运用苦柯胺干预白细胞介素1β诱导小鼠软骨细胞炎症和铁死亡体外模型,以及建立内侧半月板损伤体内骨关节炎模型发现,苦柯胺显著减轻了软骨细胞炎症,苦柯胺显著上调了SIRT1、Nrf2、HO-1和GPX4的表达,且SIRT1抑制剂EX-527能够逆转苦柯胺的抗炎和抗铁死亡作用,表明苦柯胺通过SIRT1/GPX4信号通路抑制软骨细胞炎症和铁死亡。在小鼠骨关节炎体内模型中,苦柯胺显著减轻了软骨损伤,进一步验证了苦柯胺对软骨细胞的保护作用。目前仅少数研究报道苦柯胺在体外和动物模型中的效果,缺乏大规模随机对照试验验证其临床疗效。
淫羊藿苷是一种类黄酮糖苷,具有强大的抗炎和抗氧化特性。XIAO等[70]运用借助白细胞介素1β诱导产生的骨关节炎体外模型和手术诱导的大鼠体内模型展开研究,发现淫羊藿苷可借助强化SLC7A11/GPX4信号通路,抑制软骨细胞发生铁死亡,减轻与骨关节炎相关的关节软骨损伤。这项研究表明,淫羊藿苷可能是一种有效的骨关节炎治疗药物,具有临床转化潜力。淫羊藿苷在临床应用方面,需要克服机制复杂性、递送效率以及临床验证等问题,未来的研究应当加大临床研究力度,以此推动其从实验室向临床的转化进程。
原阿片碱(Protopine)是一种生物碱,具有强大的抗炎和抗氧化活性。CHEN等[71]运用原阿片碱干预通过叔丁基过氧化氢处理小鼠软骨细胞骨关节炎体外模型及前交叉韧带横断小鼠体内模型后得出结论,原阿片碱可以通过Nrf2通路抑制软骨细胞铁死亡及炎症反应,为骨关节炎治疗提供了新方向。原阿片碱的潜力在于其多靶点作用,但需解决从实验室到临床的转化。未来需要结合精准医学和新型递送技术,可能使其成为骨关节炎治疗中兼具症状缓解和疾病修饰潜力的候选药物。
鲁斯可皂苷元(Ruscogenin)是一种麦冬中分离的生物活性化合物,具有抗炎和抗氧化活性。研究发现,鲁斯可皂苷元可以通过抑制铁死亡来抑制叶酸诱导的肾损伤[72]。RUAN等[73]用白细胞介素1β刺激小鼠软骨细胞,模拟骨关节炎的炎症环境,研究发现鲁斯可皂苷元能够激活Nrf2信号通路,增加SLC7A11和GPX4的表达,从而抑制铁死亡。当Nrf2被敲低时,鲁斯可皂苷元的抗炎和抗铁死亡作用被逆转。在半月板失稳手术诱导的小鼠体外骨关节炎模型中,鲁斯可皂苷元能够减轻骨关节炎小鼠的软骨破坏,表明鲁斯可皂苷元在体内也具有保护作用。因此得出结论,鲁斯可皂苷元可通过Nrf2/SLC7A11/GPX4信号通路抑制软骨细胞铁死亡,缓解骨关节炎的进展。当前关于鲁斯可皂苷元的研究数据主要基于细胞以及动物模型,缺少人体临床试验来验证其安全性和有效性,未来应当开展临床试验,重点评估其对骨关节炎患者疼痛、功能评分以及影像学进展的影响。
长春西汀是长春花植物衍生的长春碱的合成衍生物,长春西汀有抗炎、抗氧化、抗凋亡以及血管舒张作用。WANG等[74]通过叔丁基过氧化氢诱导软骨细胞铁死亡体外模型发现,长春西汀有效减轻了软骨细胞细胞外基质的降解以及线粒体损伤,并且通过激活Nrf2/GPX4通路抑制软骨细胞铁死亡。而在半月板失稳手术诱导的小鼠骨关节炎体内模型中,长春西汀明显改善了小鼠软骨变性以及骨赘形成,缓解了骨关节炎的发展。长春西汀的骨关节炎治疗潜力受限于机制尚未明确以及临床证据不足,未来需要开展临床转化研究。
茶黄素-3,3′-二加拉酸酯(TF3)是一种从红茶中提取的天然多酚化合物,具有抗氧化和抗炎特性。XU等[75]研究发现,TF3可逆转 Erastin 诱导的软骨细胞活力下降,且可以通过激活 Nrf2/GPX4 信号通路显著抑制软骨细胞铁死亡,Nrf2敲低或GPX4抑制剂处理均削弱了TF3的保护作用,因此,TF3通过激活Nrf2/GPX4信号通路发挥作用,缓解骨关节炎进展。TF3在临床应用转化中也存在着药物递送效率低、安全性证据不足等问题,未来需进一步加强研究机制解析、递送技术革新和严谨的临床试验设计,以验证其作为骨关节炎治疗药物的潜力。
京尼平苷酸是从杜仲中提取的生物活性化合物,具有抑制炎症和抗氧化的作用,SUN等[76]运用白细胞介素1β刺激小鼠软骨细胞模拟骨关节炎的炎症环境,观察京尼平苷酸对炎症介质和铁死亡相关指标的影响,结果发现,京尼平苷酸减轻了白细胞介素1β诱导的软骨细胞活力下降及胞内丙二醛和铁的产生,同时增加了GSH、GPX4、Nrf2和HO-1的表达,Nrf2抑制剂或Nrf2敲低能够逆转京尼平苷酸对炎症和铁死亡的抑制作用,表明京尼平苷酸通过激活Nrf2信号通路发挥其保护作用。因此得出结论,京尼平苷酸通过激活Nrf2/GPX4信号通路抑制炎症和软骨细胞的铁死亡,缓解骨关节炎的进展。目前对京尼平苷酸的研究主要集中在京尼平苷酸对软骨细胞的保护作用和在动物模型中的作用效果,缺乏对京尼平苷酸在人类关节组织中代谢和靶向性的深入研究。
短叶老鹤草素A(Brevilin A)是从药草石胡荽(Centipeda minima)中分离的生物活性化合物,其有抗炎、抗肿瘤等诸多生物活性。RUAN等[77]研究发现,短叶老鹤草素A可显著抑制白细胞介素1β刺激的软骨细胞炎症反应。此外,短叶老鹤草素A还可以通过调节SIRT1/Nrf2/GPX4信号传导通路抑制软骨细胞铁死亡,缓解骨关节炎的进展。短叶老鹤草素A的骨关节炎治疗潜力受限于临床验证不足,未来需优先解决从动物模型到人类应用的转化挑战。
甾体皂苷元(Sarsasapogenin)是一种在植物根茎中提取的天然化合物,具有抗炎、抗氧化等多种药理作用。Yes相关蛋白1(Yes-associated protein 1,YAP1) 是人体内有转录激活功能的蛋白,在调节细胞增殖以及细胞分化方面发挥着重要作用[78]。CHEN等[79]在研究中发现,甾体皂苷元可抑制白细胞介素1β诱导的软骨细胞铁死亡,具体表现为GPX4和SLC7A11表达上调,ACSL4表达下调。在失稳半月板诱导产生的骨关节炎大鼠模型中,关节内注射甾体皂苷元治疗使软骨退化和骨赘形成明显减轻,并且显著改善了软骨下骨的微结构。此外,甾体皂苷元还可通过上调YAP1的表达来抑制软骨细胞铁死亡,YAP1被敲低后,甾体皂苷元的保护作用会被削弱,而YAP1过表达则会提高甾体皂苷元的抗铁死亡效果。未来需进一步加强临床研究,已验证其安全性及疗效。
棉酚乙酸是棉酚的一种药用形式,近年来棉酚乙酸的多种生物活性,包括抗炎和抗肿瘤作用已被广泛报道。有研究表明,GPX4 DNA甲基化可以使GPX4表达下调并促进细胞铁死亡的发展[80]。SHANG等[81]的实验表明,棉酚乙酸通过抑制GPX4甲基化减轻骨关节炎中软骨细胞的铁死亡。但棉酚乙酸在体内代谢可能引起肝功能异常[82],尤其在长期使用时风险增加。因此,未来需要通过研究合成衍生物减少不良反应,同时保留其抗炎和软骨保护活性,且进一步加强临床研究,以确认其安全性及疗效。
苏木酮A(Sappanone A)是一种黄酮类天然化合物,具有抗炎、抗氧化等多种生物活性。ZHANG等[83]的研究聚焦于该化合物对骨关节炎相关病理机制的影响,以白细胞介素1β 诱导的软骨细胞为体外模型,同时构建大鼠骨关节炎动物模型,系统探究其作用机制。在体外实验中,苏木酮 A 展现出显著的软骨细胞保护效应,一方面,有效缓解了白细胞介素1β引发的炎症反应;另一方面,通过激活 SIRT1/Nrf2信号通路,促使细胞内活性氧、丙二醛和铁离子水平显著下降,同时显著提升GSH水平,并上调 GPX4 表达,从而有效抑制软骨细胞发生铁死亡。在动物实验层面,关节内注射苏木酮A后,大鼠骨关节炎模型的软骨破坏程度明显减轻,软骨细胞铁死亡得到有效抑制,这一结果与体外实验形成呼应,进一步证实了SIRT1/Nrf2信号通路在苏木酮 A 抑制铁死亡过程中的关键作用。综合体内外研究结果,苏木酮A可通过激活 SIRT1/Nrf2 信号通路抑制软骨细胞铁死亡,进而减轻骨关节炎的炎症反应,减缓细胞外基质的降解进程。值得注意的是,目前关于苏木酮A的研究多局限于体外细胞实验与动物模型研究,尚未开展大规模随机对照临床试验。因此,苏木酮A在人体中的治疗效果与安全性仍需更严谨的临床研究加以验证,这也是未来该领域研究亟待解决的重要方向。
槲皮素作为广泛存在于果蔬及植物中的天然黄酮类化合物,近年来在疾病防治领域展现出显著的多效性。RUAN等[84]通过白细胞介素1β诱导的软骨细胞模型发现,槲皮素能够剂量依赖性地抑制炎症递质及细胞外基质降解酶的表达,同时促进Ⅱ型胶原蛋白的合成。进一步机制研究表明,槲皮素可上调SIRT1、Nrf2及HO-1蛋白表达水平,揭示其通过激活SIRT1/Nrf2/HO-1信号通路有效抑制软骨细胞铁死亡进程。在动物实验层面,研究人员采用前交叉韧带切断术构建大鼠骨关节炎模型,发现槲皮素干预不仅显著减轻了关节疼痛行为学评分,还能改善软骨组织病理学改变,抑制软骨下骨的异常重塑;生化检测显示,槲皮素可显著降低血清中炎症因子水平,同时减少细胞外基质降解标志物含量,证实了其对骨关节炎进展的抑制作用。然而,槲皮素的临床应用受限于其极低的水溶性和肠道吸收效率,KARIM等[85]开发的纳米颗粒载药系统通过提升槲皮素的细胞摄取效率,在衰老软骨细胞模型中显著增强了铁过载清除能力。值得注意的是,当前研究仍主要集中于细胞和动物实验阶段,缺乏高质量临床研究数据支撑。未来研究需重点突破3个方向:开发新型水溶性衍生物以改善药代动力学特性;构建靶向递药系统提升组织特异性;开展多中心随机对照临床试验验证其安全性与有效性。
丹参酮ⅡA作为中药丹参的主要活性成分之一,在多系统疾病治疗领域展现出重要价值。XU等[86]构建脂多糖诱导的软骨细胞骨关节炎体外模型,并给予不同浓度丹参酮ⅡA进行干预,结果表明丹参酮ⅡA 能显著抑制脂多糖诱导的软骨细胞凋亡,有效减缓细胞外基质降解进程。通过机制探究发现,该化合物可显著降低细胞内活性氧、丙二醛水平以及游离铁离子浓度,同时上调GSH含量和GPX4表达。基于上述实验数据推断,丹参酮ⅡA可能通过抑制铁死亡通路,发挥改善软骨细胞生存状态、延缓软骨退变的作用,进而对骨关节炎的疾病进展产生积极影响。值得注意的是,目前丹参酮ⅡA治疗骨关节炎的相关研究多局限于细胞实验和动物模型层面,在人体临床应用中的有效性和安全性仍需大样本、多中心的临床试验进一步验证。
柚皮素是一种天然黄酮类化合物,具有多种生物活性,如抗氧化、抗炎、抗癌等。PAN等[87]研究发现,在白细胞介素1β和柠檬酸铁胺诱导的软骨细胞骨关节炎体外实验里,柚皮素可在一定程度上减少软骨细胞中的铁离子积累,抑制因铁过载引发的线粒体膜电位下降,起到保护线粒体功能的效果;柚皮素还能显著上调Nrf2和HO-1的表达,抑制软骨细胞氧化应激;当使用Nrf2抑制剂时,柚皮素的保护作用便会被抑制。而在失稳半月板手术诱导的骨关节炎小鼠体内实验中,柚皮素可减轻小鼠的软骨损伤以及软骨下骨增生状况,减少滑膜炎以及关节软骨的磨损现象。以上实验结果表明,柚皮素通过抑制氧化应激和激活Nrf2/HO-1通路,减轻铁过载诱导的软骨损伤,显示出其在骨关节炎治疗中的潜在应用价值。但柚皮素存在水溶性差、口服吸收率低等问题,导致血药浓度不足,WANG等[88]设计了聚己内酯/聚乙二醇-柚皮素纳米纤维膜的 pH 响应系统,使柚皮素能够在骨关节炎模型的弱酸性环境中持续释放,以此提升了药物递送效率,增强了柚皮素的疗效。但目前柚皮素治疗骨关节炎的研究中仍存在长期毒性等问题,因此未来需进一步开展大规模临床试验来完成实验室向临床的转化。
D-甘露糖是一种天然存在于多种植物中的单糖,在生物学及医学领域中具有多种重要作用。目前已有研究表明,缺氧诱导因子2α在调节骨关节炎进展中具有重要作用,缺氧诱导因子2α表达异常可能通过影响软骨细胞代谢、增殖与分化来参与骨关节炎的发展[89]。ZHOU等[90]通过研究发现,缺氧诱导因子2α在D-甘露糖诱导的软骨细胞铁死亡抵抗中起重要作用。过表达缺氧诱导因子2α消除了D-甘露糖的软骨保护功效,并抵消了D-甘露糖作为铁死亡抑制剂的作用。由此可见,D-甘露糖通过抑制缺氧诱导因子2α介导的软骨细胞铁死亡敏感性,缓解了骨关节炎的进展。
多种铁死亡抑制剂在治疗骨关节炎中的作用机制见表3。

| [1] GLYN-JONES S, PALMER AJR, AGRICOLA R, et al. Osteoarthritis. Lancet (London, England). 2015;386(9991):376-387. [2] COURTIES A, KOUKI I, SOLIMAN N, et al. Osteoarthritis year in review 2024: Epidemiology and therapy. Osteoarthritis Cartilage. 2024;32(11):1397-1404. [3] ZHENG L, ZHANG Z, SHENG P, et al. The role of metabolism in chondrocyte dysfunction and the progression of osteoarthritis. Age Res Rev. 2021;66:101249. [4] LU S, LIU Z, QI M, et al. Ferroptosis and its role in osteoarthritis: mechanisms, biomarkers, and therapeutic perspectives. Front Cell Dev Biol. 2024;12:1510390. [5] YANG J, HU S, BIAN Y, et al. Targeting Cell Death: Pyroptosis, Ferroptosis, Apoptosis and Necroptosis in Osteoarthritis. Front Cell Dev Biol. 2021;9:789948. [6] DIXON SJ, LEMBERG KM, LAMPRECHT MR, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012; 149(5):1060-1072. [7] DIXON SJ, OLZMANN JA. The cell biology of ferroptosis. Nat Rev Mol Cell Biol. 2024; 25(6):424-442. [8] LEPETSOS P, PAPAVASSILIOU AG. ROS/oxidative stress signaling in osteoarthritis. Biochimica Et Biophysica Acta. 2016; 1862(4):576-591. [9] YAO X, SUN K, YU S, et al. Chondrocyte ferroptosis contribute to the progression of osteoarthritis. J Orthop Trans. 2021;27:33-43. [10] MIAO Y, CHEN Y, XUE F, et al. Contribution of ferroptosis and GPX4’s dual functions to osteoarthritis progression. EBioMedicine. 2022;76:103847. [11] GUNSHIN H, MACKENZIE B, BERGER UV, et al. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388(6641):482-488. [12] HENTZE MW, MUCKENTHALER MU, GALY B, et al. Two to tango: regulation of Mammalian iron metabolism. Cell. 2010; 142(1):24-38. [13] RU Q, LI Y, CHEN L, et al. Iron homeostasis and ferroptosis in human diseases: mechanisms and therapeutic prospects. Signal Transduct Target Ther. 2024;9(1):271. [14] YIN H, XU L, PORTER NA. Free radical lipid peroxidation: mechanisms and analysis. Chem Rev. 2011;111(10):5944-5972. [15] ROCHETTE L, DOGON G, RIGAL E, et al. Lipid Peroxidation and Iron Metabolism: Two Corner Stones in the Homeostasis Control of Ferroptosis. Int J Mol Sci. 2022;24(1): 449. [16] AYALA A, MUÑOZ MF, ARGÜELLES S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid Med Cell Longev. 2014;2014:360438. [17] LIU J, KANG R, TANG D. Signaling pathways and defense mechanisms of ferroptosis. FEBS J. 2022;289(22):7038-7050. [18] LIU Y, WAN Y, JIANG Y, et al. GPX4: The hub of lipid oxidation, ferroptosis, disease and treatment. Biochim Biophys Acta Rev Cancer. 2023;1878(3):188890. [19] DODSON M, CASTRO-PORTUGUEZ R, ZHANG DD. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019;23:101107. [20] ZHAO X, LI M, LU Y, et al. Sirt1 inhibits macrophage polarization and inflammation in gouty arthritis by inhibiting the MAPK/NF-κB/AP-1 pathway and activating the Nrf2/HO-1 pathway. Inflamm Res. 2024;73(7): 1173-1184. [21] JIANG L, HICKMAN JH, WANG SJ, et al. Dynamic roles of p53-mediated metabolic activities in ROS-induced stress responses. Cell Cycle. 2015;14(18): 2881-2885. [22] LAI L, TAN M, HU M, et al. Important molecular mechanisms in ferroptosis. Mol Cell Biochem. 2025;480(2):639-658. [23] BELLOTTI D, REMELLI M. Deferoxamine B: A Natural, Excellent and Versatile Metal Chelator. Molecules (Basel, Switzerland). 2021;26(11):3255. [24] MEHANA ESE, KHAFAGA AF, EL-BLEHI SS. The role of matrix metalloproteinases in osteoarthritis pathogenesis: An updated review. Life Sci. 2019;234:116786. [25] GUO Z, LIN Y, LIU H, et al. Deferoxamine alleviates chondrocyte senescence and osteoarthritis progression by maintaining iron homeostasis. Int Immunopharmacol. 2024;139:112619. [26] GUO Z, LIN J, SUN K, et al. Deferoxamine Alleviates Osteoarthritis by Inhibiting Chondrocyte Ferroptosis and Activating the Nrf2 Pathway. Front Pharmacol. 2022;13: 791376. [27] PANEBIANCO R, VIALE M, VECCHIO G. Development of Deferoxamine-Functionalized Cyclodextrin Polymer for Targeted Iron Chelation. Chem Open. 2025:e2500186. doi: 10.1002/open.202500186. [28] GHARANIZADEH K, SHARIFI AM, TAYYEBI H, et al. Core decompression combined with local DFO administration loaded on polylactic glycolic acid scaffolds for the treatment of osteonecrosis of the femoral head: a pilot study. BMC Pharmacol Toxicol. 2023;24(1):44. [29] ZENG L, YANG T, YANG K, et al. Efficacy and Safety of Curcumin and Curcuma longa Extract in the Treatment of Arthritis: A Systematic Review and Meta-Analysis of Randomized Controlled Trial. Front Immunol. 2022;13:891822. [30] JIAO Y, WILKINSON J, CHRISTINE PIETSCH E, et al. Iron chelation in the biological activity of curcumin. Free Rad Biol Med. 2006; 40(7):1152-1160. [31] JIN Z, CHANG B, WEI Y, et al. Curcumin exerts chondroprotective effects against osteoarthritis by promoting AMPK/PINK1/Parkin-mediated mitophagy. Biomed Pharmacother. 2022;151:113092. [32] ZHOU Y, JIA Z, WANG J, et al. Curcumin reverses erastin-induced chondrocyte ferroptosis by upregulating Nrf2. Heliyon. 2023;9(10):e20163. [33] 陈凡, 周富丽, 陈勇, 等.姜黄素可能通过上调Prdx6蛋白表达抑制软骨细胞铁死亡[J]. 安徽医科大学学报,2023,58(12): 2106-2112. [34] ZENG L, YU G, HAO W, et al. The efficacy and safety of Curcuma longa extract and curcumin supplements on osteoarthritis: a systematic review and meta-analysis. Biosci Rep. 2021;41(6):BSR20210817. [35] SHENG W, LI A, YUE Y, et al. A Novel Curcumin-Loaded Nanoplatform Alleviates Osteoarthritis by Inhibiting Chondrocyte Ferroptosis. Macromol Rapid Commun. 2025;46(7):e2400495. [36] GUAN T, DING LG, LU BY, et al. Combined Administration of Curcumin and Chondroitin Sulfate Alleviates Cartilage Injury and Inflammation via NF-κB Pathway in Knee Osteoarthritis Rats. Front Pharmacol. 2022; 13:882304. [37] WANG X, LIU Z, PENG P, et al. Astaxanthin attenuates osteoarthritis progression via inhibiting ferroptosis and regulating mitochondrial function in chondrocytes. Chem Biol Interact. 2022;366:110148. [38] XU W, ZHANG B, XI C, et al. Ferroptosis Plays a Role in Human Chondrocyte of Osteoarthritis Induced by IL-1β In Vitro. Cartilage. 2023;14(4):455-466. [39] ZHANG NY, LIU JY, ZHENG H, et al. Design, Synthesis, and Biological Evaluation of New Improved Ferrostatin-1 Derived Ferroptosis Inhibitors. Chem Biodiv. 2025;22(2): e202402141. [40] KRAINZ T, GASCHLER MM, LIM C, et al. A Mitochondrial-Targeted Nitroxide Is a Potent Inhibitor of Ferroptosis. ACS Cent Sci. 2016; 2(9):653-659. [41] SUN W, LV Z, LI W, et al. XJB-5-131 protects chondrocytes from ferroptosis to alleviate osteoarthritis progression via restoring Pebp1 expression. J Orthop Translat. 2024; 44:114-124. [42] CHEN X, ZHANG B, LIU T, et al. Liproxstatin-1 Attenuates Morphine Tolerance through Inhibiting Spinal Ferroptosis-like Cell Death. ACS Chem Neurosci. 2019;10(12):4824-4833. [43] CHENG B, ZHANG J, SHEN Q, et al. Liproxstatin-1 alleviates cartilage degradation by inhibiting chondrocyte ferroptosis in the temporomandibular joint. Biol Cell. 2024;116(1):e202300042. [44] HE R, WEI Y, PENG Z, et al. α-Ketoglutarate alleviates osteoarthritis by inhibiting ferroptosis via the ETV4/SLC7A11/GPX4 signaling pathway. Cell Mol Biol Lett. 2024;29(1):88. [45] WANG X, XUE Y, HAO K, et al. Sustained therapeutic effects of self-assembled hyaluronic acid nanoparticles loaded with α-Ketoglutarate in various osteoarthritis stages. Biomaterials. 2025;314:122845. [46] GALANO A, REITER RJ. Melatonin and its metabolites vs oxidative stress: From individual actions to collective protection. J Pineal Res. 2018;65(1):e12514. [47] PARK MW, CHA HW, KIM J, et al. NOX4 promotes ferroptosis of astrocytes by oxidative stress-induced lipid peroxidation via the impairment of mitochondrial metabolism in Alzheimer’s diseases. Redox Biol. 2021;41:101947. [48] WANG Q, QI B, SHI S, et al. Melatonin Alleviates Osteoarthritis by Regulating NADPH Oxidase 4-Induced Ferroptosis and Mitigating Mitochondrial Dysfunction. J Pineal Res. 2024;76(6):e12992. [49] LI H, ZHOU B, WU J, et al. Melatonin is a potential novel analgesic agent for osteoarthritis: Evidence from cohort studies in humans and preclinical research in rats. J Pineal Res. 2024;76(2):e12945. [50] LIANG H, YAN Y, SUN W, et al. Preparation of Melatonin-Loaded Nanoparticles with Targeting and Sustained Release Function and Their Application in Osteoarthritis. Int J Mol Sci. 2023;24(10):8740. [51] EIYAMA A, OKAMOTO K. PINK1/Parkin-mediated mitophagy in mammalian cells. Curr Opin Cell Biol. 2015;33:95-101. [52] HOU L, WANG G, ZHANG X, et al. Mitoquinone alleviates osteoarthritis progress by activating the NRF2-Parkin axis. iScience. 2023;26(9):107647. [53] HUOVINEN J, PALOSAARI S, PESONEN P, et al. 1,25(OH)2D3 and its analogue calcipotriol inhibit the migration of human synovial and mesenchymal stromal cells in a wound healing model - A comparison with glucocorticoids. J Steroid Biochem Mol Biol. 2023;233:106373. [54] ZHAI G, DORÉ J, RAHMAN P. TGF-β signal transduction pathways and osteoarthritis. Rheumatol Int. 2015;35(8):1283-1292. [55] YANG Z, JIANG W, XIONG C, et al. Calcipotriol suppresses GPX4-mediated ferroptosis in OA chondrocytes by blocking the TGF-β1 pathway. Cytokine. 2023;171:156382. [56] LI Y, FENG D, WANG Z, et al. Ischemia-induced ACSL4 activation contributes to ferroptosis-mediated tissue injury in intestinal ischemia/reperfusion. Cell Death Differ. 2019;26(11):2284-2299. [57] DAI Y, CHEN Y, MO D, et al. Inhibition of ACSL4 ameliorates tubular ferroptotic cell death and protects against fibrotic kidney disease. Commun Biol. 2023;6(1):907. [58] CAO S, WEI Y, YUE Y, et al. Rosiglitazone retards the progression of iron overload-induced osteoarthritis by impeding chondrocyte ferroptosis. iScience. 2024; 27(9):110526. [59] JIN ZL, GAO WY, LIAO SJ, et al. Paeonol inhibits the progression of intracerebral haemorrhage by mediating the HOTAIR/UPF1/ACSL4 axis. ASN Neuro. 2021;13: 17590914211010647. [60] CAO S, WEI Y, XIONG A, et al. Paeonol inhibits ACSL4 to protect chondrocytes from ferroptosis and ameliorates osteoarthritis progression. J Orthop Trans. 2025;50:1-13. [61] BIHLET AR, BYRJALSEN I, ANDERSEN JR, et al. The efficacy and safety of a fixed-dose combination of apocynin and paeonol, APPA, in symptomatic knee OA: A double-blind, randomized, placebo-controlled, clinical trial. Osteoarthritis Cartilage. 2024; 32(7):952-962. [62] GONG Z, WANG Y, LI L, et al. Cardamonin alleviates chondrocytes inflammation and cartilage degradation of osteoarthritis by inhibiting ferroptosis via p53 pathway. Food Chem Toxicol. 2023;174:113644. [63] YANG Y, LIU Y, WANG Y, et al. Regulation of SIRT1 and Its Roles in Inflammation. Front Immunol. 2022;13:831168. [64] LI S, HE Q, CHEN B, et al. Cardamonin protects against iron overload induced arthritis by attenuating ROS production and NLRP3 inflammasome activation via the SIRT1/p38MAPK signaling pathway. Sci Rep. 2023;13(1):13744. [65] HE Q, YANG J, PAN Z, et al. Biochanin A protects against iron overload associated knee osteoarthritis via regulating iron levels and NRF2/System xc-/GPX4 axis. Biomed Pharmacother. 2023;157:113915. [66] WANG X, LIU Z, PENG P, et al. Astaxanthin attenuates osteoarthritis progression via inhibiting ferroptosis and regulating mitochondrial function in chondrocytes. Chemico. 2022;366:110148. [67] STONEHOUSE W, BENASSI-EVANS B, BEDNARZ J, et al. Krill oil improved osteoarthritic knee pain in adults with mild to moderate knee osteoarthritis: a 6-month multicenter, randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2022;116(3):672-685. [68] WAN Y, SHEN K, YU H, et al. Baicalein limits osteoarthritis development by inhibiting chondrocyte ferroptosis. Free Radical Biol Med. 2023;196:108-120. [69] SUN J, ZHANG Y, WANG C, et al. Kukoamine A protects mice against osteoarthritis by inhibiting chondrocyte inflammation and ferroptosis via SIRT1/GPX4 signaling pathway. Life Sci. 2023;332:122117. [70] XIAO J, LUO C, LI A, et al. Icariin inhibits chondrocyte ferroptosis and alleviates osteoarthritis by enhancing the SLC7A11/GPX4 signaling. Int Immunopharmacol. 2024;133:112010. [71] CHEN H, ZHONG Y, SANG W, et al. Protopine protects chondrocytes from undergoing ferroptosis by activating Nrf2 pathway. Biochem Biophys Res Commun. 2024;710: 149599. [72] HU M, AN S. Ruscogenin Prevents Folic Acid-Induced Acute Kidney Damage by Inhibiting Rev-erbα/β-Mediated Ferroptosis. Comput Intell Neurosci. 2022;2022:8066126. [73] RUAN Q, WANG C, ZHANG Y, et al. Ruscogenin attenuates cartilage destruction in osteoarthritis through suppressing chondrocyte ferroptosis via Nrf2/SLC7A11/GPX4 signaling pathway. Chemico. 2024; 388:110835. [74] WANG J, YANG J, FANG Y, et al. Vinpocetine protects against osteoarthritis by inhibiting ferroptosis and extracellular matrix degradation via activation of the Nrf2/GPX4 pathway. Phytomedicine. 2024;135: 156115. [75] XU C, NI S, XU N, et al. Theaflavin-3,3’-Digallate Inhibits Erastin-Induced Chondrocytes Ferroptosis via the Nrf2/GPX4 Signaling Pathway in Osteoarthritis. Oxid Med Cell Longev. 2022;2022:3531995. [76] SUN J, SONG X, WANG C, et al. Geniposidic acid alleviates osteoarthritis progression through inhibiting inflammation and chondrocytes ferroptosis. J Cell Mol Med. 2024;28(8):e18228. [77] RUAN Q, WANG C, ZHANG Y, et al. Brevilin A attenuates cartilage destruction in osteoarthritis mouse model by inhibiting inflammation and ferroptosis via SIRT1/Nrf2/GPX4 signaling pathway. Int Immunopharmacol. 2023;124(Pt B): 110924. [78] WANG J, ZHU Q, LI R, et al. YAP1 protects against septic liver injury via ferroptosis resistance. Cell Biosci. 2022;12:163. [79] CHEN R, YING C, ZOU Y, et al. Sarsasapogenin inhibits YAP1-dependent chondrocyte ferroptosis to alleviate osteoarthritis. Biomed Pharmacother. 2023;168:115772. [80] WANG X, KONG X, FENG X, et al. Effects of DNA, RNA, and Protein Methylation on the Regulation of Ferroptosis. Int J Biol Sci. 2023;19(11):3558-3575. [81] SHANG J, XIONG C, JIANG W, et al. Gossypol Acetic Acid alleviates the Ferroptosis of Chondrocytes in Osteoarthritis by Inhibiting GPX4 Methylation. Curr Med Chem. 2024. doi: 10.2174/0109298673280730231211092905. [82] YU J, YANG H, WANG J, et al. Comprehensive analysis of histophysiology, transcriptomics and metabolomics in goslings exposed to gossypol acetate: unraveling hepatotoxic mechanisms. Front Vet Sci. 2025;12: 1527284. [83] ZHANG Z, ZHANG N, LI M, et al. Sappanone a alleviates osteoarthritis progression by inhibiting chondrocyte ferroptosis via activating the SIRT1/Nrf2 signaling pathway. Naunyn. 2024;397(11):8759-8770. [84] RUAN H, ZHU T, WANG T, et al. Quercetin Modulates Ferroptosis via the SIRT1/Nrf-2/HO-1 Pathway and Attenuates Cartilage Destruction in an Osteoarthritis Rat Model. Int J Mol Sci. 2024;25(13):7461. [85] KARIM A, QAISAR R, SURESH S, et al. Nanoparticle-delivered quercetin exhibits enhanced efficacy in eliminating iron-overloaded senescent chondrocytes. Nanomedicine (London, England). 2024; 19(26):2159-2170. [86] XU J, ZHI X, ZHANG Y, et al. Tanshinone IIA alleviates chondrocyte apoptosis and extracellular matrix degeneration by inhibiting ferroptosis. Open Life Sciences. 2023;18(1):20220666. [87] PAN Z, HE Q, ZENG J, et al. Naringenin protects against iron overload-induced osteoarthritis by suppressing oxidative stress. Phytomedicine. 2022;105:154330. [88] WANG Z, ZHONG Y, HE S, et al. Application of the pH-Responsive PCL/PEG-Nar Nanofiber Membrane in the Treatment of Osteoarthritis. Front Bioeng Biotechnol. 2022;10:859442. [89] SAITO T, KAWAGUCHI H. HIF-2α as a possible therapeutic target of osteoarthritis. Osteoarthritis Cartilage. 2010;18(12):1552-1556. [90] ZHOU X, ZHENG Y, SUN W, et al. D‐mannose alleviates osteoarthritis progression by inhibiting chondrocyte ferroptosis in a HIF‐2α‐dependent manner. Cell Prolif. 2021; 54(11):e13134. |
| [1] | 杨学涛, 朱梦菡, 张宸熙, 孙一民, 叶 玲. 抗氧化纳米材料在口腔中的应用和不足[J]. 中国组织工程研究, 2026, 30(8): 2044-2053. |
| [2] | 刘大为, 崔颖颖, 王方辉, 王子轩, 陈宇涵, 李友瑞, 张荣和. 表没食子儿茶素没食子酸酯介导活性氧双向调控及在纳米材料中的应用[J]. 中国组织工程研究, 2026, 30(8): 2101-2112. |
| [3] | 王菘芃, 刘玉三, 于焕英, 高晓丽, 徐英江, 张晓明, 刘 敏. 沸石基咪唑盐框架8纳米材料的活性氧双向调控:从肿瘤治疗、抗菌到细胞保护[J]. 中国组织工程研究, 2026, 30(8): 2033-2013. |
| [4] | 邹玉莲, 陈朝沛, 黄海霞, 兰玉燕, 刘 敏, 黄 婷. 白藜芦醇在炎症微环境下促进骨髓间充质干细胞的成骨分化[J]. 中国组织工程研究, 2026, 30(7): 1669-1678. |
| [5] | 李 豪, 陶红成, 曾 平, 刘金富, 丁 强, 牛驰程, 黄 凯, 康宏誉. 丝裂原活化蛋白激酶信号通路调控骨关节炎的发生发展:指导中药靶点治疗[J]. 中国组织工程研究, 2026, 30(6): 1476-1485. |
| [6] | 温广伟, 甄颖豪, 郑泰铿, 周淑怡, 莫国业, 周腾鹏, 李海山, 赖以毅. 异银杏素对破骨细胞分化的影响和机制[J]. 中国组织工程研究, 2026, 30(6): 1348-1358. |
| [7] | 吕国庆, 艾孜麦提江·肉孜, 熊道海. 鸢尾素抑制人关节软骨细胞中铁死亡的作用及其机制[J]. 中国组织工程研究, 2026, 30(6): 1359-1367. |
| [8] | 胡 静, 朱 伶, 谢 娟, 孔德营, 刘豆豆. 自噬影响组蛋白修饰标记H3K4me3调控小鼠早期胚胎发育[J]. 中国组织工程研究, 2026, 30(5): 1147-1155. |
| [9] | 阴勇成, 赵相瑞, 杨志杰, 李 政, 李 芳, 宁 斌. 过氧化物还原酶1在脊髓损伤后小胶质细胞炎症反应中的作用及机制[J]. 中国组织工程研究, 2026, 30(5): 1106-1113. |
| [10] | 邹荣基, 喻芳芳, 王茂林, 贾卓鹏 . 雷公藤内酯酮抑制铁死亡改善大脑动脉闭塞/再灌注模型大鼠脑缺血再灌注损伤[J]. 中国组织工程研究, 2026, 30(4): 873-881. |
| [11] | 杨 肖, 白月辉, 赵甜甜, 王东昊, 赵 琛, 袁 硕. 颞下颌关节骨关节炎软骨退变:机制及再生的挑战[J]. 中国组织工程研究, 2026, 30(4): 926-935. |
| [12] | 郑 雯, 朱东生, 王晓东. 分泌型模块化钙结合蛋白调控发育性髋关节发育不良大鼠髋臼软骨细胞自噬[J]. 中国组织工程研究, 2026, 30(18): 4618-4626. |
| [13] | 于 乐, 南淞华, 史子剑, 和琪琪, 李振家, 崔应麟 . 线粒体自噬、铁死亡、铜死亡与双硫死亡在帕金森病中的作用机制[J]. 中国组织工程研究, 2026, 30(17): 4446-4456. |
| [14] | 刘安南, 李建辉, 高 伟, 李 雪, 宋 婧, 邢丽萍, 李虹霖. 铁死亡与阿尔茨海默病的文献计量学分析[J]. 中国组织工程研究, 2026, 30(16): 4278-4288. |
| [15] | 龚玉康, 叶高祺, 王晨浩, 陈德金, 高文山. 天然多酚基水凝胶促进骨修复的作用与机制[J]. 中国组织工程研究, 2026, 30(14): 3675-3686. |
铁死亡是一种新型的铁依赖性细胞死亡方式,与传统形式的细胞死亡如细胞凋亡、焦亡、自噬、衰老和坏死性凋亡等完全不同,具有独特的形态学、生化、遗传和免疫学特征[5]。这种新型细胞死亡方式是由DIXON等[6]于2012年首次提出,他发现有一种小分子化合物erastin诱导的细胞死亡与其他细胞死亡方式不同,这种细胞死亡方式是由于细胞内脂质过氧化物的含量以铁依赖的方式异常增加而造成细胞死亡,因此把它命名为“铁死亡”。细胞铁死亡在形态上表现出独特的线粒体结构异常,包括线粒体萎缩、线粒体嵴减少和线粒体膜密度增加[7]。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
1.1 资料来源
1.1.1 检索人及检索时间 第一作者于2025年1月进行检索。
1.1.2 检索文献时限 2012年1月至 2025年1月。
1.1.3 检索数据库 中国知网和PubMed数据库。
1.1.4 检索词 中文检索词为“骨关节炎,铁死亡,脂质过氧化,铁死亡抑制剂,软骨细胞,活性氧,谷胱甘肽过氧化物酶4”,英文检索词为“osteoarthritis,ferroptosis,lipid peroxidation,ferroptosis inhibitors,chondrocytes,reactive oxygen species,glutathione peroxidase 4”。
1.1.5 检索文献类型 综述、临床试验和研究原著。
1.1.6 手工检索情况 无。
1.1.7 检索策略 以PubMed数据库为例,见图1。
1.2 入组标准
1.2.1 纳入标准 ①铁死亡与骨关节炎相关的文章;②铁死亡抑制剂在软骨细胞发挥调控作用的文章。
1.2.2 排除标准 与入选标准无关,不符合研究目的的文献;重复报道和无参考意义的文献。
1.3 文献质量评估和数据提取 数据库综合检索到5 000余篇相关文献,按照入选标准进行标题及摘要初步筛查,排除重复、陈旧和与主题无关的文献,最终通过详细阅读纳入90篇文献,其中89篇来源于PubMed数据库,1篇来源于中国知网。仔细阅读文章内容,总结与文章相符的有效信息进行总结归纳,见图2。
3.1 既往他人在该领域研究的贡献和存在的问题 骨关节炎作为常见的慢性退行性关节疾病,其病理机制涉及复杂的细胞生物学和分子生物学过程。尽管近年来围绕骨关节炎的发病机制研究取得了显著进展,但关节软骨退变、滑膜炎症等核心病理进程的分子调控网络仍存在诸多未解之谜。值得关注的是,铁死亡作为一种依赖铁离子介导的程序性细胞死亡新形式,正逐渐成为骨关节炎发病机制研究的热点领域。
最新研究证据表明,铁死亡相关调控通路在骨关节炎病理进程中发挥关键作用。例如,System Xc-/GSH/GPX4轴通过维持细胞内氧化还原稳态,调节软骨细胞存活与凋亡平衡;MAPK/核因子κB信号通路激活可诱导炎症因子释放,加剧关节微环境损伤;p53依赖性铁死亡途径参与软骨细胞衰老与代谢紊乱;而Nrf2抗氧化防御系统则通过调控铁代谢和脂质过氧化水平,影响骨关节炎的疾病进展。这些发现为揭示骨关节炎病理机制提供了新视角,也为靶向铁死亡开发治疗策略奠定了理论基础。
然而,当前研究仍存在显著局限性:一方面,铁死亡在骨关节炎中的分子调控机制尚未完全阐明,特别是在疾病不同阶段(早期软骨退变、中期滑膜炎症、晚期关节结构破坏)及不同细胞类型(软骨细胞、滑膜细胞、巨噬细胞等)中的特异性作用机制仍需深入探究;另一方面,尽管铁死亡抑制剂在体外细胞模型和动物实验中展现出良好的治疗效果,但其临床转化仍面临诸多挑战。现有研究多为单中心、小样本的基础实验,缺乏大规模、多中心临床试验验证其安全性和有效性;此外,药物剂量优化、给药途径选择以及长期用药安全性等问题均需系统性研究,以推动铁死亡靶向治疗从实验室走向临床应用。
3.2 作者综述区别于他人他篇的特点 文章系统梳理了铁死亡在骨关节炎病理进程中的作用机制。通过对System Xc-/GSH/GPX4、MAPK/NF-κB、p53及Nrf2等关键信号通路的深入剖析,揭示了铁死亡可通过调控细胞氧化还原平衡、炎症反应、细胞衰老与代谢等多个维度,参与骨关节炎的发生发展。研究发现,铁死亡相关信号通路的异常激活或抑制,不仅影响软骨细胞的生存状态,还能通过改变关节微环境,促进软骨退变和滑膜炎症,为阐释骨关节炎复杂病理机制提供了新的理论依据。
同时,文章对当前铁死亡抑制剂的研究进展进行了全面综述。依据作用靶点和作用方式的差异,将铁死亡抑制剂分为靶向铁代谢、脂质过氧化、抗氧化系统等多个类别,并详细阐述了各抑制剂的作用机制。结果表明,这些抑制剂能够通过多靶点、多途径阻断铁死亡进程,在细胞和动物模型中展现出显著的骨关节炎治疗潜力,其多样性的作用模式不仅体现了铁死亡调控网络的复杂性,更为后续开发高效、特异性的骨关节炎治疗药物提供了重要参考。此次研究成果有望为铁死亡在骨关节炎领域的深入研究及相关抑制剂的临床转化提供理论支撑与实践指导。
3.3 综述的局限性 ①目前多数关于铁死亡抑制剂的研究还处在细胞和动物实验阶段,缺少大规模且多中心的临床试验数据用以验证其安全性与有效性,虽然这些抑制剂在实验室里呈现出不错的治疗效果,不过它们在临床应用中的效果以及安全性仍有待验证。②铁死亡在骨关节炎里的具体分子机制还未完全阐明,在不同骨关节炎阶段以及不同细胞类型中的作用机制仍需进一步研究。现有的研究主要聚焦于软骨细胞的铁死亡,而其他细胞类型比如滑膜细胞、成骨细胞等在骨关节炎中的作用尚未得到充分研究。③铁死亡抑制剂的作用机制多样,涉及多个信号通路和分子靶点,尽管这种多样性为治疗提供了多种可能性,但也增加了研究的复杂性。不同抑制剂之间的相互作用、剂量效应以及潜在的不良作用仍需进一步研究。
3.4 综述的重要意义 此次综述在揭示铁死亡在骨关节炎中的关键作用、提供新的治疗靶点、推动铁死亡抑制剂临床应用相关领域研究进展以及提高公众健康意识等方面具有重要意义,不仅为骨关节炎的治疗提供了新的思路和方法,也为未来的研究和临床应用奠定了坚实的基础。
3.5 课题组专家对未来的建议 目前铁死亡在骨关节炎中的具体分子机制尚未完全阐明,未来的研究应进一步探索铁死亡于不同骨关节炎阶段、不同细胞类型中的作用机制,以及铁死亡与其他细胞死亡方式(如凋亡、自噬等)的相互作用。同时,未来应开展大规模临床试验,推动铁死亡抑制剂从实验室研究向临床应用的转变,探索更有效的给药方式如关节内注射、纳米递送系统等,以提升药物在靶组织里的稳定性与活性。此外,铁死亡抑制剂在短期实验中显示出良好的治疗效果,但长期使用的安全性和潜在的不良作用仍未阐明。因此,未来需要深入研究铁死亡抑制剂对铁代谢、抗氧化系统等的长期影响,评估其潜在的不良作用和风险。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
骨关节炎是一种常见的慢性退行性关节疾病,主要表现为关节软骨的退行性变、滑膜炎症和骨质增生。近年来,随着对铁死亡这一新型细胞死亡方式的深入研究,铁死亡在骨关节炎中的发病机制中的作用逐渐被揭示。铁死亡抑制剂通过多种机制、多种信号通路抑制铁死亡,展现出在骨关节炎治疗中的广阔前景。但目前大多数研究仍处于细胞和动物实验阶段,缺乏大规模临床试验验证其安全性和有效性。未来的研究应进一步探索铁死亡的具体机制,并推动铁死亡抑制剂的临床应用,为骨关节炎的治疗提供新的策略。本文详细描述了铁死亡对骨关节炎的作用机制,及多种铁死亡抑制剂在治疗骨关节炎中的作用。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||