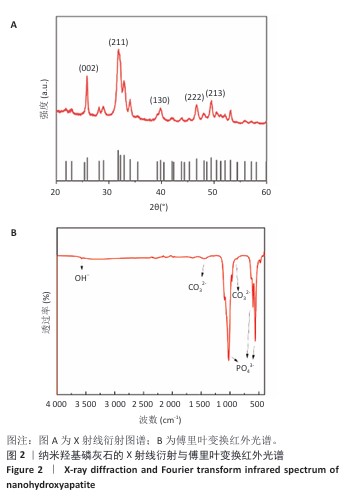
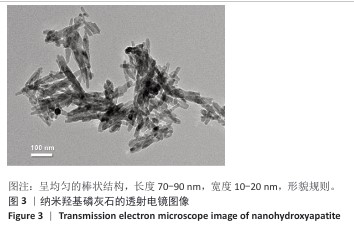
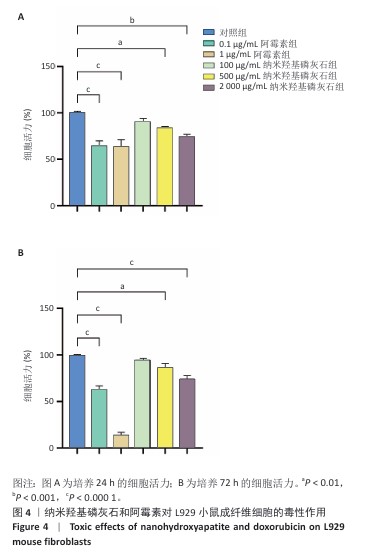
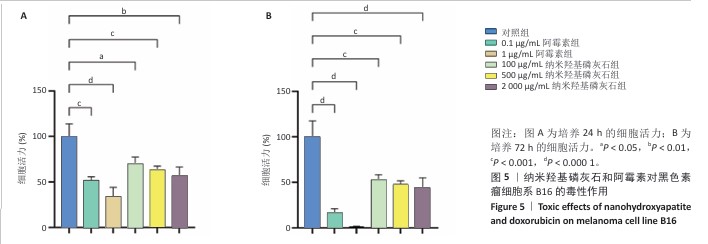
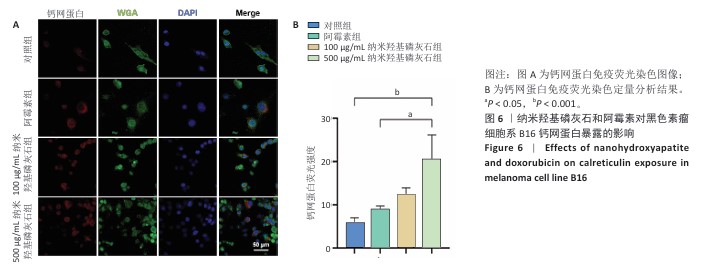
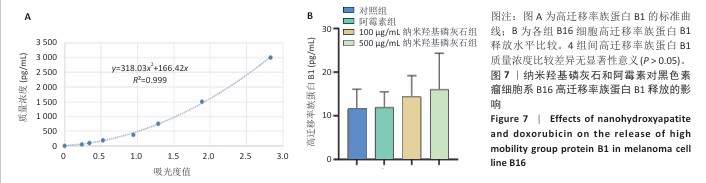
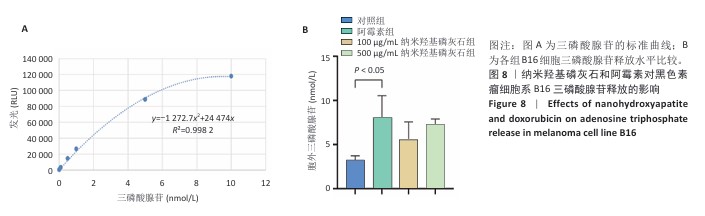
[1] BRAY F, LAVERSANNE M, SUNG H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229-263.
[2] BRAY F, LAVERSANNE M, WEIDERPASS E, et al. The ever‐increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127(16):3029-3030.
[3] SCHIRRMACHER V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment (Review). Int J Oncol. 2018;54(2):407-419.
[4] AHMED A, TAIT SWG. Targeting immunogenic cell death in cancer. Mol Oncol. 2020;14(12):2994-3006.
[5] ZHOU J, WANG G, CHEN Y, et al. Immunogenic cell death in cancer therapy: Present and emerging inducers. J Cell Mol Med. 2019;23(8): 4854-4865.
[6] KROEMER G, GALASSI C, ZITVOGEL L, et al. Immunogenic cell stress and death. Nat Immunol. 2022;23(4):487-500.
[7] YAN C, ZHAO Y, LIU X, et al. Self-Delivery Nanobooster to Enhance Immunogenic Cell Death for Cancer Chemoimmunotherapy. ACS Appl Mater Interfaces. 2024;16(26):33169-33181.
[8] FENG X, LIN T, CHEN D, et al. Mitochondria-associated ER stress evokes immunogenic cell death through the ROS-PERK-eIF2α pathway under PTT/CDT combined therapy. Acta Biomater. 2023;160:211-224.
[9] BROOKES PS, YOON Y, ROBOTHAM JL, et al. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004; 287(4):C817-833.
[10] ZHENG P, DING B, JIANG Z, et al. Ultrasound-Augmented Mitochondrial Calcium Ion Overload by Calcium Nanomodulator to Induce Immunogenic Cell Death. Nano Lett. 2021;21(5):2088-2093.
[11] VAN VLIET AR, AGOSTINIS P. Mitochondria-Associated Membranes and ER Stress. Coordinating Organismal Physiology Through the Unfolded Protein Response. 2017:73-102.
[12] YE L, ZENG Q, LING M, et al. Inhibition of IP3R/Ca2+ Dysregulation Protects Mice From Ventilator-Induced Lung Injury via Endoplasmic Reticulum and Mitochondrial Pathways. Front Immunol. 2021;12: 729094.
[13] MAKIO T, CHEN J, SIMMEN T. ER stress as a sentinel mechanism for ER Ca2+ homeostasis. Cell Calcium. 2024;124:102961.
[14] XU Q, CHEN C, LIN A, et al. Endoplasmic reticulum stress-mediated membrane expression of CRT/ERp57 induces immunogenic apoptosis in drug-resistant endometrial cancer cells. Oncotarget. 2017;8(35): 58754-58764.
[15] ZHU M, WANG T, WANG H, et al. LW-213 induces immunogenic tumor cell death via ER stress mediated by lysosomal TRPML1. Cancer Lett. 2023;577:216435.
[16] ABDUL HALIM NA, HUSSEIN MZ, KANDAR MK. Nanomaterials-Upconverted Hydroxyapatite for Bone Tissue Engineering and a Platform for Drug Delivery. Int J Nanomedicine. 2021;16:6477-6496.
[17] BHAT S, UTHAPPA UT, ALTALHI T, et al. Functionalized Porous Hydroxyapatite Scaffolds for Tissue Engineering Applications: A Focused Review. ACS Biomater Sci Eng. 2021;8(10):4039-4076.
[18] GEORGE SM, NAYAK C, SINGH I, et al. Multifunctional Hydroxyapatite Composites for Orthopedic Applications: A Review. ACS Biomater Sci Eng. 2022;8(8):3162-3186.
[19] CHEN S, XING Z, GENG M, et al. Macrophage fusion event as one prerequisite for inorganic nanoparticle-induced antitumor response. Sci Adv. 2023;9(29):eadd9871.
[20] ZHAO H, WU C, GAO D, et al. Antitumor Effect by Hydroxyapatite Nanospheres: Activation of Mitochondria-Dependent Apoptosis and Negative Regulation of Phosphatidylinositol-3-Kinase/Protein Kinase B Pathway. ACS Nano. 2018;12(8):7838-7854.
[21] WANG J, WU Y, LI H, et al. Antitumor effects of polydopamine coated hydroxyapatite nanoparticles and its mechanism: Mitochondria-targeted ROS and calcium channels. Biomater Adv. 2024;161:213858.
[22] GUO G, TIAN A, LAN X, et al. Nano hydroxyapatite induces glioma cell apoptosis by suppressing NF κB signaling pathway. Exp Ther Med. 2019;17(5):4080-4088.
[23] LI Z, TANG J, WU H, et al. A systematic assessment of hydroxyapatite nanoparticles used in the treatment of melanoma. Nano Res. 2020; 13(8):2106-2117.
[24] YIN M, YIN Y, CHEN H, et al. Effect of Hydroxyapatite Nanoparticles on the Growth Potential of Hepatoma Cells in Nude Mice. J Nanosci Nanotechnol. 2015;15(5):3816-3822.
[25] YANG Y, YANG J, ZHU N, et al. Tumor-targeting hydroxyapatite nanoparticles for remodeling tumor immune microenvironment (TIME) by activating mitoDNA-pyroptosis pathway in cancer. J Nanobiotechnology. 2023;21(1):470.
[26] CHEN Q, PENG B, LIN L, et al. Chondroitin Sulfate‐Modified Hydroxyapatite for Caspase‐1 Activated Induced Pyroptosis through Ca Overload/ER Stress/STING/IRF3 Pathway in Colorectal Cancer. Small. 2024;20(43):e2403201.
[27] MEENA R, KESARI KK, RANI M, et al. Effects of hydroxyapatite nanoparticles on proliferation and apoptosis of human breast cancer cells (MCF-7). J Nanopart Res. 2012;14(2):712.
[28] TANG W, YUAN Y, LIU C, et al. Differential Cytotoxicity and Particle Action of Hydroxyapatite Nanoparticles in Human Cancer Cells. Nanomedicine. 2014;9(3):397-412.
[29] DONG X, SUN Y, LI Y, et al. Synergistic Combination of Bioactive Hydroxyapatite Nanoparticles and the Chemotherapeutic Doxorubicin to Overcome Tumor Multidrug Resistance. Small. 2021; 17(18):e2007672.
[30] DONG X, ZANG C, SUN Y, et al. Hydroxyapatite nanoparticles induced calcium overload-initiated cancer cell-specific apoptosis through inhibition of PMCA and activation of calpain. J Mater Chem B. 2023; 11(32):7609-7622.
[31] ZHANG H, LIU R, WAN P, et al. Targeting tumor energy metabolism via simultaneous inhibition of mitochondrial respiration and glycolysis using biodegradable hydroxyapatite nanorods. Colloids Surf B Biointerfaces. 2023;226:113330.
[32] HUA Y, WU J, WU H, et al. Exposure to hydroxyapatite nanoparticles enhances Toll-like receptor 4 signal transduction and overcomes endotoxin tolerance in vitro and in vivo. Acta Biomater. 2021;135:650-662.
[33] HORNBECK PV. Enzyme‐Linked Immunosorbent Assays. Curr Protoc Immunol. 2015;110:2.1.1-2.1.23.
[34] DAI E, ZHU Z, WAHED S, et al. Epigenetic modulation of antitumor immunity for improved cancer immunotherapy. Mol Cancer. 2021; 20(1):171.
[35] SUEK N, CAMPESATO LF, MERGHOUB T, et al. Targeted APC Activation in Cancer Immunotherapy to Enhance the Abscopal Effect. Front Immunol. 2019;10:604.
[36] GALLUZZI L, VITALE I, WARREN S, et al. Consensus guidelines for the definition, detection and interpretation of immunogenic cell death. J Immunother Cancer. 2020;8(1):e000337.
[37] DUAN X, CHAN C, LIN W. Nanoparticle‐Mediated Immunogenic Cell Death Enables and Potentiates Cancer Immunotherapy. Angew Chem Int Ed Engl. 2018;58(3): 670-680.
[38] FUCIKOVA J, KEPP O, KASIKOVA L, et al. Detection of immunogenic cell death and its relevance for cancer therapy. Cell Death Dis. 2020; 11(11):1013.
[39] KRYSKO O, LØVE AAES T, BACHERT C, et al. Many faces of DAMPs in cancer therapy. Cell Death Dis. 2013;4(5):e631.
[40] OBEID M, TESNIERE A, GHIRINGHELLI F, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2006;13(1):54-61.
[41] ZHAO L, LIU P, KEPP O, et al. Methods for measuring HMGB1 release during immunogenic cell death. Tumor Immunology and Immunotherapy – Molecular Methods. 2019:177-193.
[42] YANG H, WANG H, ANDERSSON U. Targeting Inflammation Driven by HMGB1. Front Immunol. 2020;11:484.
[43] GOUGEON ML, MELKI MT, SAÏDI H. HMGB1, an alarmin promoting HIV dissemination and latency in dendritic cells. Cell Death Differ. 2011;19(1):96-106.
[44] DUBYAK GR, EL-MOATASSIM C. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am J Physiol. 1993;265(3):C577-C606.
[45] FORVEILLE S, HUMEAU J, SAUVAT A, et al. Quinacrine-mediated detection of intracellular ATP. Methods Enzymol. 2019;629:103-113.
[46] HIRANO S, ZHOU Q, FURUYAMA A, et al. Differential Regulation of IL-1β and IL-6 Release in Murine Macrophages. Inflammation. 2017;40(6):1933-1943.
[47] XIE Q, SHEN WW, ZHONG J, et al. Lipopolysaccharide/adenosine triphosphate induces IL-1β and IL-18 secretion through the NLRP3 inflammasome in RAW264.7 murine macrophage cells. Int J Mol Med. 2014;34(1):341-349. |