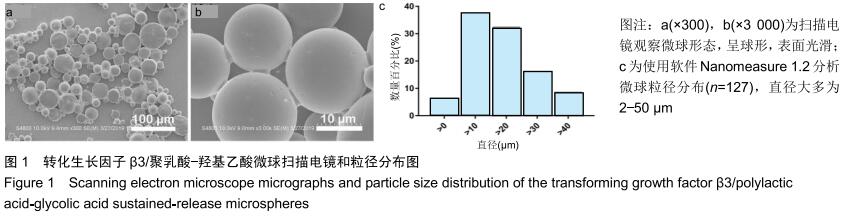
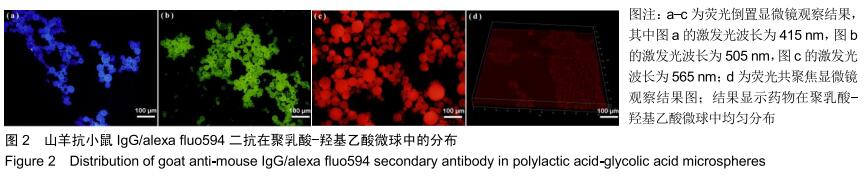
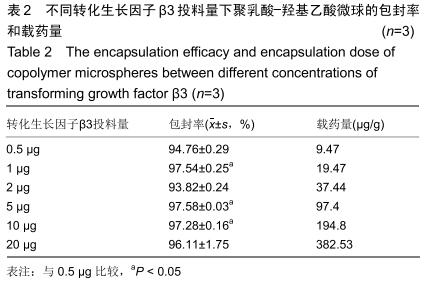
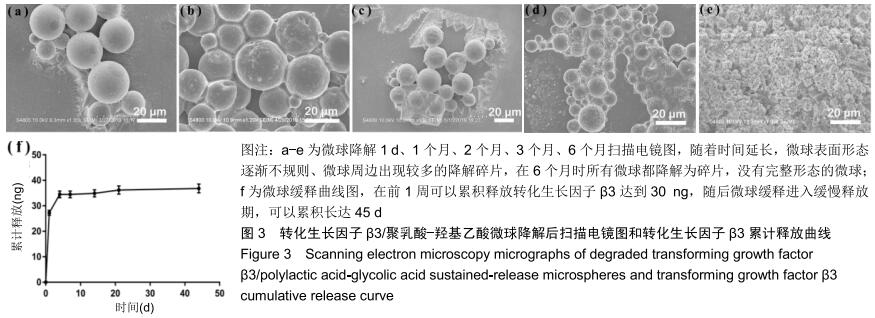
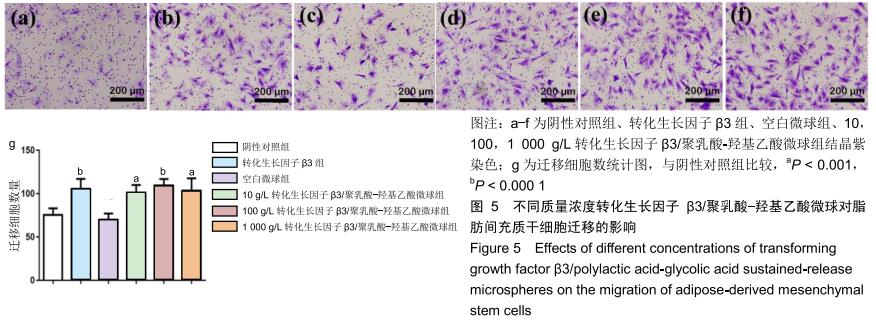
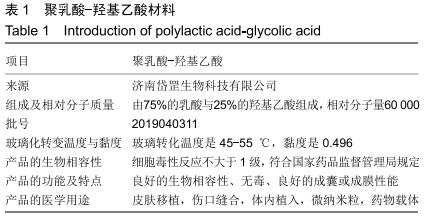
|
[1] MORILLE M, TOUPET K, MONTERO-MENEI CN, et al. PLGA-based microcarriers induce mesenchymal stem cell chondrogenesis and stimulate cartilage repair in osteoarthritis. Biomaterials. 2016;88:60-69.
[2] BUCKWALTER JA, MANKIN HJ. Articular cartilage: degeneration and osteoarthritis, repair, regeneration, and transplantation.Instr Course Lect.1998;47:487-504.
[3] CHEN D, SHEN J, ZHAO W, et al. Osteoarthritis: toward a comprehensive understanding of pathological mechanism. Bone Res.2017;5:16044.
[4] VAN OSCH GJ, BRITTBERG M, DENNIS JE, et al. Cartilage repair: past and future–lessons for regenerative medicine. J Cell Mol Med.2009;13(5):792-810.
[5] WANG J, SUN B, TIAN L, et al. Evaluation of the potential of rhTGF-β3 encapsulated P (LLA-CL)/collagen nanofibers for tracheal cartilage regeneration using mesenchymal stems cells derived from Wharton's jelly of human umbilical cord. Mater Sci Eng C.2017;70:637-645.
[6] CHEN H, SUN J, WANG Z, et al. Magnetic Cell–Scaffold Interface Constructed by Superparamagnetic IONP Enhanced Osteogenesis of Adipose-Derived Stem Cells. ACS Appl Mater Interfaces. 2018;10(51):44279-44289.
[7] JING H, GAO B, GAO M, et al. Restoring tracheal defects in a rabbit model with tissue engineered patches based on TGF-β3-encapsulating electrospun poly (l-lactic acid-co-ε-caprolactone)/collagen scaffolds. Artif Cell Nanomed Biotechnol.2018;46(sup1):985-995.
[8] YANG Q, TENG BH, WANG LN, et al. Silk fibroin/cartilage extracellular matrix scaffolds with sequential delivery of TGF-β3 for chondrogenic differentiation of adipose-derived stem cells. Int J Nanomed. 2017; 12: 6721.
[9] LEE CH, COOK JL, MENDELSON A,et al.Regeneration of the articular surface of the rabbit synovial joint by cell homing: a proof of concept study.Lancet.2010; 376(9739): 440-448.
[10] LIANG C, LI H, TAO Y, et al. Dual release of dexamethasone and TGF-β3 from polymeric microspheres for stem cell matrix accumulation in a rat disc degeneration model. Acta Biomater. 2013; 9(12): 9423-9433.
[11] DORMER NH, GUPTA V, SCURTO AM, et al. Effect of different sintering methods on bioactivity and release of proteins from PLGA microspheres.Mater Sci Eng C. 2013; 33(7):4343-4351.
[12] PARK JS, PARK K, WOO DG, et al. PLGA microsphere construct coated with TGF-β 3 loaded nanoparticles for neocartilage formation. Biomacromolecules.2008; 9(8): 2162-2169.
[13] ALI M, WALBOOMERS XF, JANSEN JA, et al. Influence of formulation parameters on encapsulation of doxycycline in PLGA microspheres prepared by double emulsion technique for the treatment of periodontitis. J Drug Deliv Sci Technol. 2019; 52: 263-271.
[14] FANG Y, ZHANG N, LI Q, et al. Characterizing the release mechanism of donepezil-loaded PLGA microspheres in vitro and in vivo.J Drug Deliv Sci Technol. 2019; 51: 430-437.
[15] HAMEED A, GALLAGHER L B, DOLAN E, et al. Insulin-like growth factor-1 (IGF-1) poly (lactic-co-glycolic acid)(PLGA) microparticles–development, characterisation, and in vitro assessment of bioactivity for cardiac applications. J Microencapsul.2019;36(3):267-277.
[16] WANG J, SUN X, ZHANG Z,et al.Silk fibroin/ collagen/hyaluronic acid scaffold incorporating pilose antler polypeptides microspheres for cartilage tissue engineering. Mater Sci Eng C.2019;94:35-44.
[17] CHENG H, ZHANG Y, ZHANG B, et al. Biocompatibility of polypropylene mesh scaffold with adipose-derived stem cells.Exp Ther Med. 2017;13(6): 2922-2926.
[18] 韩爽,卢世璧,刘强,等.自体脂肪间充质干细胞复合人脐带Wharton胶支架修复兔膝关节软骨缺损[J].中国组织工程研究, 2012,16(19):3496-3501.
[19] GOLDRING MB, TSUCHIMOCHI K,IJIRI K. The control of chondrogenesis. J Cell Biochem. 2006; 97(1): 33-44.
[20] CLEARY MA, VAN OSCH GJ, BRAMA PA, et al. FGF, TGFβ and Wnt crosstalk: embryonic to in vitro cartilage development from mesenchymal stem cells. J Tissue Eng Regen Med. 2015; 9(4): 332-342.
[21] GREEN JD, TOLLEMAR V, DOUGHERTY M, et al. Multifaceted signaling regulators of chondrogenesis: implications in cartilage regeneration and tissue engineering. Genes Dis. 2015;2(4):307-327.
[22] SEYEDIN SM, THOMAS TC, THOMPSON AY, et al. Purification and characterization of two cartilage-inducing factors from bovine demineralized bone.Proc Natl Acad Sci U S A. 1985;82(8):2267-2271.
[23] HEINE UL, MUNOZ EF, FLANDERS KC, et al. Role of transforming growth factor-beta in the development of the mouse embryo.J Cell Biol.1987; 105(6):2861-2876.
[24] ROSEN DM, STEMPIEN SA, THOMPSON AY, et al. Differentiation of rat mesenchymal cells by cartilage-inducing factor: Enhanced phenotypic expression by dihydrocytochalasin B. Exp Cell Res.1986; 165(1): 127-138.
[25] ALMEIDA HV, ESWARAMOORTHY R, CUNNIFFE GM, et al. Fibrin hydrogels functionalized with cartilage extracellular matrix and incorporating freshly isolated stromal cells as an injectable for cartilage regeneration. Acta Biomater.2016;36: 55-62.
[26] BIAN L, ZHAI DY, TOUS E, et al. Enhanced MSC chondrogenesis following delivery of TGF-β3 from alginate microspheres within hyaluronic acid hydrogels in vitro and in vivo. Biomaterials. 2011; 32(27): 6425-6434.
[27] WEISS S, HENNIG T, BOCK R, et al. Impact of growth factors and PTHrP on early and late chondrogenic differentiation of human mesenchymal stem cells.J Cell Physiol.2010; 223(1): 84-93.
[28] MARTINS C, SOUSA F, ARAUJO F, et al. Functionalizing PLGA and PLGA derivatives for drug delivery and tissue regeneration applications. Adv Healthc Mater.2018; 7(1): 1701035.
[29] MIR M, AHMED N, REHMAN AU. Recent applications of PLGA based nanostructures in drug delivery.Colloids Surf B Biointerfaces. 2017; 159: 217-231.
[30] YAO S, LIU H, YU S, et al. Drug-nanoencapsulated PLGA microspheres prepared by emulsion electrospray with controlled release behavior.Regen Biomater.2016;3(5):309-317.
[31] DENG Y, SUN AX, OVERHOLT KJ, et al. Enhancing chondrogenesis and mechanical strength retention in physiologically relevant hydrogels with incorporation of hyaluronic acid and direct loading of TGF-β. Acta Biomater. 2019;83:167-176.
[32] ZHOU M, LOZANO N, WYCHOWANIEC JK, et al. Graphene oxide: a growth factor delivery carrier to enhance chondrogenic differentiation of human mesenchymal stem cells in 3D hydrogels.Acta Biomater.2019; 96: 271-280.
[33] SANCHO-TELLO M, FORRIOL F, MARTÍN DE LLANO JJ, et al. Biostable scaffolds of polyacrylate polymers implanted in the articular cartilage induce hyaline-like cartilage regeneration in rabbits.Int J Artif Organs.2017;40(7): 350-357.
[34] SUN X, WANG J, WANG Y, et al. Collagen-based porous scaffolds containing PLGA microspheres for controlled kartogenin release in cartilage tissue engineering. Artif Cell Nanomed Biotechnol.2018;46(8):1957-1966.
[35] LUO Z, JIANG L, XU Y, et al. Mechano growth factor (MGF) and transforming growth factor (TGF)-β3 functionalized silk scaffolds enhance articular hyaline cartilage regeneration in rabbit model. Biomaterials.2015;52:463-475.
|