Chinese Journal of Tissue Engineering Research ›› 2023, Vol. 27 ›› Issue (21): 3391-3397.doi: 10.12307/2023.420
Previous Articles Next Articles
Effects of scaffolds on angiogenic microenvironment and its mechanism
Zhang Yi, Ren Sicong, Huangfu Huimin, Xu Jing, Yang Zhen, Zhou Yanmin
- Department of Oral Implantology, Hospital of Stomatology, Jilin University, Changchun 130021, Jilin Province, China
-
Received:2022-06-08Accepted:2022-07-21Online:2023-07-28Published:2022-11-24 -
Contact:Zhou Yanmin, Professor, Chief physician, Doctoral supervisor, Department of Oral Implantology, Hospital of Stomatology, Jilin University, Changchun 130021, Jilin Province, China -
About author:Zhang Yi, Master candidate, Department of Oral Implantology, Hospital of Stomatology, Jilin University, Changchun 130021, Jilin Province, China
CLC Number:
Cite this article
Zhang Yi, Ren Sicong, Huangfu Huimin, Xu Jing, Yang Zhen, Zhou Yanmin. Effects of scaffolds on angiogenic microenvironment and its mechanism[J]. Chinese Journal of Tissue Engineering Research, 2023, 27(21): 3391-3397.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
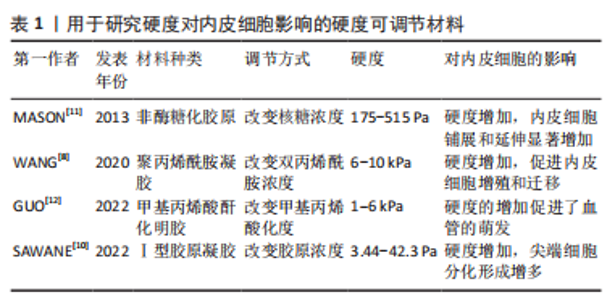
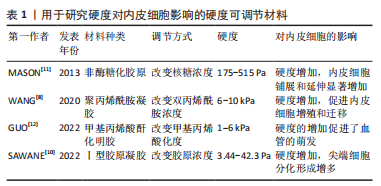
2.1 生物物理调控 血管内皮细胞具有机械响应性,能通过一系列机械感受器感知细胞外基质的生物物理信号,然后通过机械转导途径转化为生化反应。目前研究发现能刺激内皮细胞的生物物理信号有:细胞外基质硬度、结构、降解性及细胞附着位点的微纳米形貌等。 2.1.1 硬度 细胞外基质硬度被认为是决定细胞命运的重要机械信号,在调节内皮细胞行为和血管生成中起关键作用。基质硬度影响内皮细胞的黏附、增殖、迁移及细胞骨架的重塑。研究表明,0.5-10 kPa范围内,内皮细胞的铺展和黏附面积随着基质硬度的增加而增加[5];但硬度过高时(25-75 kPa),内皮细胞的黏附力反而会降低[6]。在血管生成过程中,内皮细胞向周围组织的迁移是形成新血管的重要步骤,而基质硬度对内皮细胞的迁移有直接影响。0.5-2.5 kPa范围内基质硬度的增加会导致内皮细胞迁移的增加和血管芽萌发的增多[7]。在这一系列过程中,磷脂酰肌醇3激酶/蛋白激酶B信号通路(PI3K/Akt)被证明发挥着重要的作用。较高的基质硬度通过激活磷脂酰肌醇3激酶/蛋白激酶B/特异性蛋白1通路(PI3K/Akt/Sp1)上调血管内皮细胞中血管内皮生长因子受体2的表达[8]。另一项研究指出,磷脂酰肌醇3激酶/蛋白激酶B/雷帕霉素靶蛋白信号通路(PI3K/Akt/mTOR)在较硬的底物上被激活,并且能够被血管内皮生长因子A刺激放大,进而增强内皮细胞增殖并分泌促血管生成因子,诱导管状结构形成[9]。 多种硬度可调节材料被用来检测内皮细胞对不同底物硬度的反应[8,10-12],见表1。"
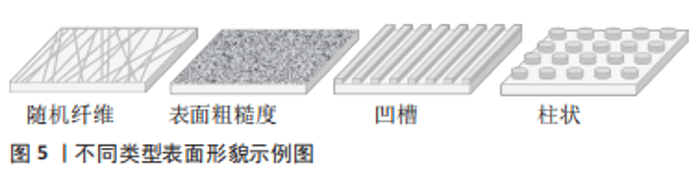
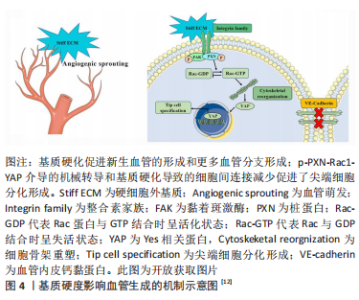
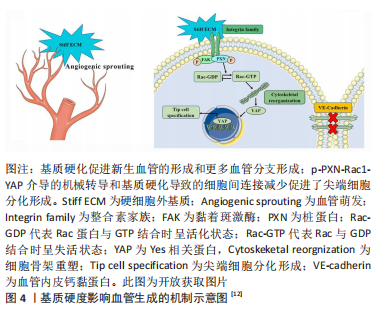
在体外,较高的基质硬度可以显著促进尖端细胞相关基因的表达以及血管的萌发和形成。机制上,刚性基质促使黏着斑激酶和桩蛋白(Paxillin,PXN)磷酸化。磷酸化后的桩蛋白(p-PXN)使细胞内活性Ras相关的C3肉毒素底物1(Rac1)水平升高,导致细胞骨架重塑和细胞硬度增加。随后,激活下游的机械效应蛋白Yes相关蛋白(Yes-associated protein,YAP)并促进其入核从而上调靶基因的表达,最终促进了尖端细胞的形成。另外,磷酸化后的桩蛋白使细胞间的连接变得松散,这也是硬度介导尖端细胞形成的原因[12]。 2.1.2 表面形貌 材料表面的微纳米形貌可以调节细胞黏附、迁移和分化等行为。出于不同的目的,多种形貌被引入,如柱、坑、凹槽、管、纤维、粗糙度和岛等[13-14],形貌示例见图5。"

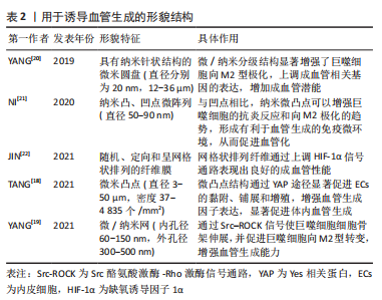
一般来说,纳米级的表面形貌决定细胞内受体分子(如整合素)的感知,影响细胞的黏附,而微米级的表面形貌影响细胞的排列和迁移。研究表明在10-100 nm尺度范围,增加材料表面的粗糙度可以增强内皮细胞黏附和生长[15],培养在平行微槽表面(宽度和间距10 μm,深度3 μm)的内皮细胞形态被拉长,细胞和肌动蛋白丝沿微槽方向排列[16]。 YAP是表面形貌影响内皮细胞行为的一个重要信号分子。研究表明,微米尺度形貌的变化刺激内皮细胞,导致Ras同源基因蛋白/Rho激酶/Ras相关的C3肉毒素底物(Rho/ROCK/Rac)依赖的细胞骨架重组和钙黏蛋白介导的细胞间连接的的丧失,从而激活YAP蛋白并促进其入核,最终促进内皮细胞增殖和迁移[17]。为了创造有利于血管化的生物物理微环境,TANG等[18]制备了具有微凸点形貌的丝素蛋白膜,膜表面的微凸点形貌提供了机械信号,通过YAP途径增强内皮细胞的黏附和增殖并提高血管内皮生长因子和碱性成纤维细胞生长因子表达水平,显著促进血管生成。 表面形貌还可以通过调节免疫微环境刺激血管生成。研究表明钛种植体表面的纳米孔形貌通过Src酪氨酸激酶-Rho激酶(Src-ROCK)信号引导巨噬细胞形态伸长并向M2型转变,构建了适宜的免疫微环境,从而增强组织成血管能力[19]。结合微米和纳米形貌的优点,YANG等[20]制备了具有微/纳米分级结构的生物陶瓷,与单一结构相比,这种分级结构表现出更好的成骨成血管潜能。文章归纳了用于诱导血管生成的形貌结构[18-22],见表2。"
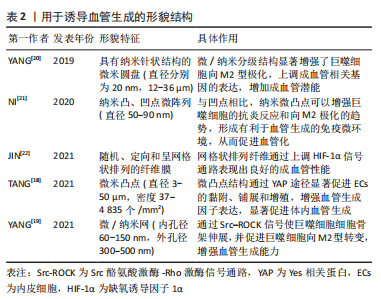
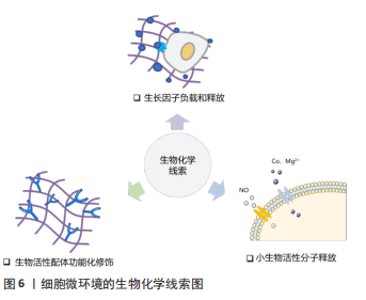
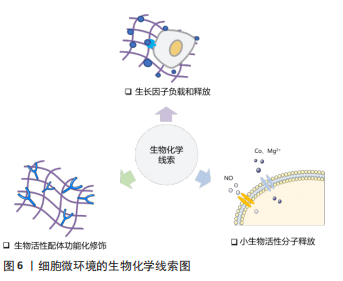
2.1.3 孔隙结构 支架材料的孔隙结构,如孔径大小、孔隙率和孔的连通性是一个重要的三维特征,显著影响细胞侵袭和血管形成。为了探究何种孔径最适宜血管化,学者们做出了诸多努力。目前认为为了优化细胞渗透、氧气和营养运输,并形成更成熟的血管化组织,支架的孔径应设计超过100 μm [23]。随着孔径的增大,生长到支架孔结构中的新血管的数量和直径都会增加。研究表明孔径为70-120 μm的壳聚糖支架有利于细胞的侵袭和增殖[24],而孔径大于200 μm的支架则可诱导直径较大的血管再生,并且新形成的血管能够更深入支架[25] 。然而支架的孔径并不是越大越好,有研究表明当孔径大于400 μm时,血管形成的程度并没有增加[26]。有学者认为支架孔径对血管化的影响主要源于材料对免疫环境的调节。研究表明较大孔径(360 μm)的胶原/壳聚糖支架促进巨噬细胞从M1向M2转化,构建了适宜血管化的免疫微环境,从而诱导更多的血管生成[27],但支架孔径介导M1向M2转变的机制尚不清楚。 孔隙率是决定血管向内生长的重要因素,基质孔隙率与内皮细胞侵入深度和萌发直径呈显著正相关[28]。孔的相互连通影响血管密度和平均侵入深度,较大的互连尺寸和高连通性能更好的促进血运重建[29-30]。ALI等[31]发现支架的孔径不同但孔连通性相似时,血管化几乎没有差异,这说明孔的连通性对血管形成的影响比孔径的更大。 2.1.4 降解性 生物材料的降解性使得其机械性能随时间发生变化,最终会影响血管生成过程。MEHDIZADEH等[32]通过计算机建模来研究支架结构变化对成血管的影响,发现在不考虑支架的结构支撑的情况下,较大的平均孔径、较高的孔隙率和快速的降解可以加速血管形成,但支架降解导致的结构支撑过早丧失会导致材料失效和血管生成中断。降解性即细胞在基质中切割出空间的速率,细胞释放蛋白酶(如基质金属蛋白酶)对基质进行主动切割从而得到形成管腔所需的空间[33]。因此LIU等[34]通过调节基质对基质金属蛋白酶的敏感度从而提高降解性,发现具有高降解性的基质能诱导更长更宽管状结构的形成。但另一项研究指出,基质的低降解性是引发内皮细胞集体迁移的关键,高降解性的基质会导致单细胞迁移[35],而多细胞迁移直接影响血管的萌发。因此在未来的研究中需要探索最合适的降解性,有利于血管萌发的同时又能满足后续管状结构形成的需要。 2.2 生物化学调控 研究表明,支架材料表面的化学功能化及材料的生物化学成分(如生长因子、离子和纳米粒子等)可以影响细胞代谢、指导细胞行为。利用这些生物化学线索,可以对成血管微环境进行调控,见图6。"
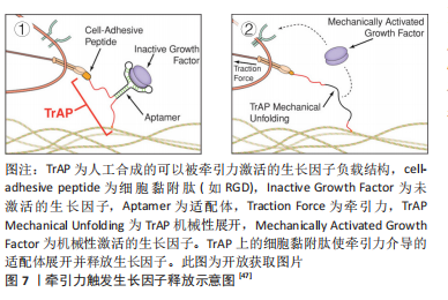
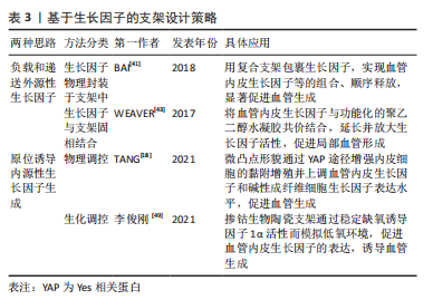
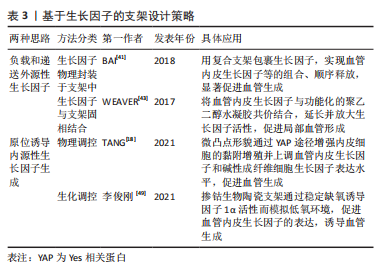
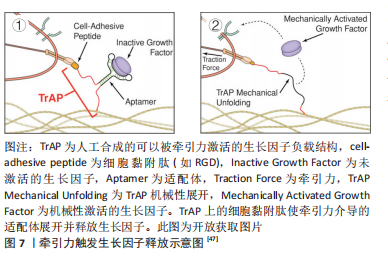
2.2.1 表面化学 细胞通过受体(如整合素和生长因子受体)识别周围的基质,因此生物材料的表面可以被不同的化学成分和分子功能化,刺激受体介导的成血管相关细胞的识别。整合素介导的细胞对细胞外基质的黏附对细胞行为和功能有重要影响。为了模拟自然状态下内皮细胞与基质间的相互作用,整合素配体精氨酸-甘氨酸-天冬氨酸(arginine-glycine-aspatic acid,RGD)肽被用来对生物材料表面进行功能化修饰[36]。RGD修饰一方面可以增加材料表面对内皮细胞的亲和力,增强内皮细胞黏附;另一方面还促进了内皮细胞增殖、迁移和管状结构形成[37]。但目前使用的RGD肽通常不具备整合素特异性,不能选择性地影响内皮细胞行为。研究表明,整合素αvβ3和α5β1在血管生成过程中有重要作用,αvβ3是血管萌发期多细胞迁移的关键调节因子,α5β1则在后期血管的形成阶段发挥作用[34]。因此,用特定整合素靶向配体对支架材料进行功能化修饰,是构建功能性血管网络的一种有前途的方法,值得进一步研究。 肝素常被用来对生物材料表面进行修饰,从而增强材料对生物活性物质的亲和力,使得生长因子(如血管内皮生长因子)能结合在材料表面以促进血管生成。MELCHIORRI等[38]对组织工程血管移植物表面进行肝素功能化修饰,从而在移植物表面固定血管内皮生长因子或抗CD34抗体。经修饰的组织工程血管移植物在体内能够显著增强内皮细胞和内皮祖细胞的附着力和活性,促进新组织形成的同时没有出现血栓或狭窄等并发症。为了更好地模拟成血管微环境,MOULISOVá等[39]用同时具有整合素和血管内皮生长因子结合位点的纤连蛋白功能化修饰聚丙烯酸乙酯片,以促进血管内皮生长因子结合,然后协同整合素/血管内皮生长因子受体信号有效促进了血管形成。 2.2.2 递送生长因子 血管生成是由多种生长因子驱动的复杂、多阶段的过程。这些生长因子不仅有多种角色,而且在不同阶段发挥协同作用,例如血管内皮生长因子、成纤维细胞生长因子2及血管生成素2在成血管初期诱导血管萌发,血小板衍生生长因子、血管生成素1则在后期稳定新生血管,导致血管成熟[40]。外源性生长因子以可溶形式给药存在半衰期短、易失活和降解以及内化速度快等问题,因此学者们希望通过生物材料实现生长因子持续、可控制的释放,使生长因子按照一定的组合和顺序在组织工程中发挥作用。传统的控释策略是将生长因子物理封装到支架材料中,通过调节基质降解性从而控制生长因子的释放动力学。BAI等[41]将血管内皮生长因子和成纤维细胞生长因子2直接整合到支架中,血小板衍生生长因子则先封装于聚乳酸微球中再掺入支架,基质间降解性的差异使血管内皮生长因子/成纤维细胞生长因子2和血小板衍生生长因子在不同阶段先后发挥作用,从而成功诱导血管生成。 研究表明,细胞外基质中的不溶性成分具有结合和保留生长因子等可溶性分子的能力,动态调节其释放、激活并呈递给细胞表面受体[42]。为了更好地模拟生长因子在生理微环境中的作用模式并增强其活性,具有固相结合生长因子能力的支架材料被开发,根据结合方式的不同,可分为共价结合和亲和力结合。将血管内皮生长因子与马来酰亚胺功能化的聚乙二醇水凝胶共价结合,可以促进局部血管形成从而提高胰岛移植物的存活率[43]。为了实现生长因子与支架材料的亲和力结合,一方面可以对支架材料表面进行功能化修饰(见结果2.2.1部分),另一方面还可以对生长因子进行工程改性。MARTINO等[44]发现结构域PlGF-2123-144具有肝素结合序列,可以与胞外基质蛋白强烈结合,于是将血管内皮生长因子、血小板衍生生长因子与该结构域融合,产生了对细胞外基质具有超亲和性的工程化生长因子变体,与原始生长因子相比,变体生长因子的组织修复能力显著增强。因此生长因子与支架材料的固相结合有助于延长并放大生长因子活性,提高生长因子利用率,从而避免过高剂量导致的副反应。 除了缓释,生长因子的递送还可以通过微环境的变化(酶、pH值和温度)和外部的刺激(超声波和光照)触发[45]。pH值作为一种反映局部血供情况的重要指标,可以作为一种刺激因素,实现生长因子的按需释放。JOSHI等[46]开发了一种可注射、pH值和温度响应性水凝胶p(NIPAAm-co-PAA-co-BA),该水凝胶在37 ℃时可以响应pH值变化(pH 7.4-6.8)从液体转变为固体,负载碱性成纤维细胞生长因子的水凝胶在弱酸性缺血环境中持续释放碱性成纤维细胞生长因子,改善局部血供;当组织恢复到生理pH值时水凝胶从局部溶解,停止释放碱性成纤维细胞生长因子,有效避免了全身副反应的产生。值得关注的是,STEJSKALOVA等[47]开发了一种方法,用细胞特定的牵引力来触发生物材料中生长因子的释放。将合成的适配体一端连接细胞黏附肽,另一端通过化学基团连接到支架材料上,适配体用来捕获血管内皮生长因子,见图7。"
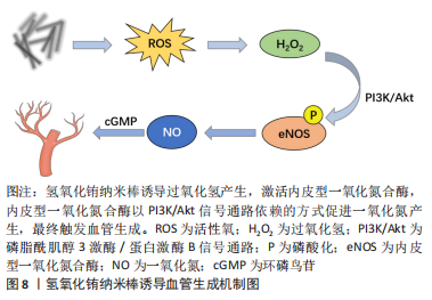
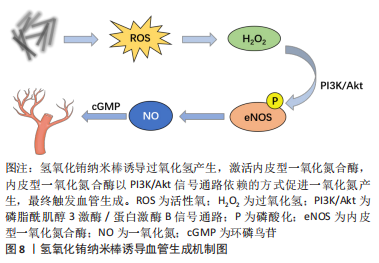
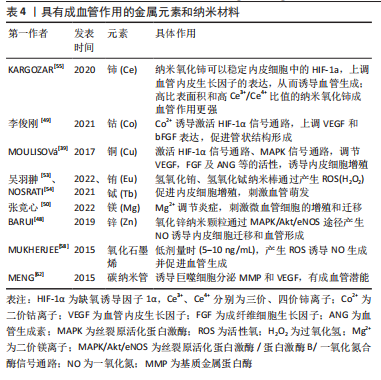
2.2.3 纳米材料 金属纳米材料:许多金属元素被认为是促进血管生成的有效因子。铜(Cu)调节成血管相关的多种蛋白和因子活性,如血管内皮生长因子、血管生成素、成纤维细胞生长因子、纤连蛋白和胶原酶等;机制上主要通过以下两条信号通路诱导血管生成:①低氧诱导的缺氧诱导因子1α通路;②丝裂原活化蛋白激酶(mitogen-activated protein kinase,MAPK)信号通路,前者涉及血管生成过程的启动,后者在内皮细胞的增殖阶段起作用[48]。钴(Co)被证明通过模拟缺氧条件来促进血管生成[49]。镁(Mg)在调节炎症反应和微血管功能方面也有直接作用[50]。因此以上元素通常以可溶性离子形式掺入生物材料发挥成血管作用。 近年来,多种金属纳米粒子作为成血管诱导剂被应用于组织工程支架中,如氧化铈、氧化锌、氧化钇、二氧化钛和氢氧化铕纳米颗粒等[51-52]。这些金属纳米颗粒能够调节植入部位的氧化还原环境,促进细胞增殖和血管生成:一方面可以通过产生活性氧并激活活性氧相关的细胞增殖途径;另一方面可以作为抗氧化剂,产生短暂的缺氧,从而导致低氧诱导的细胞通路的表达。氢氧化铕和氢氧化铽纳米棒被证明在体外促进人脐静脉内皮细胞增殖,在体内刺激血管的萌发[53],其作用机制与活性氧有关,它们可以诱导过氧化氢产生,激活内皮型一氧化氮合酶,内皮型一氧化氮合酶以PI3K/Akt信号通路依赖性方式促进一氧化氮产生,最终触发血管生成[54],见图8。"
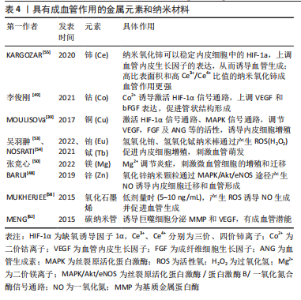
氧化锌纳米颗粒则被证明通过MAPK/Akt/内皮型一氧化氮合酶途径产生一氧化氮来诱导内皮细胞迁移和血管形成[48]。而氧化铈纳米颗粒主要通过稳定内皮细胞中缺氧诱导因子1α的表达发挥成血管诱导作用,并且高比表面积和高三价铈离子/四价铈离子比值能够增强对细胞内氧含量的催化活性,从而导致更强的促血管生成作用[55]。金纳米粒子也表现出一定的成血管潜能,在光生物调节疗法中使用金纳米粒子可以促进血管生成、上皮细胞增殖和胶原形成,加速伤口的愈合[56]。金纳米粒子还可以作为药物输送系统来输送血管内皮生长因子以促进血管生成[57]。 碳基纳米材料:非金属碳基纳米材料在调控血管生成方面也发挥重要作用。氧化石墨烯是一种经化学修饰的二维石墨烯材料。研究表明,氧化石墨烯和还原氧化石墨烯在较低剂量时(5-10 ng/mL)表现出促血管生成特性,但在高剂量时(≥50 ng/mL) 却有抑制作用。机制上,氧化石墨烯诱导细胞产生活性氧,影响蛋白激酶B磷酸化,进而促进一氧化氮合酶磷酸化,导致细胞内一氧化氮水平升高,最终触发血管生成[58]。QIAN等[59]利用3D打印技术开发了一种氧化石墨烯/聚己内酯纳米支架,发现氧化石墨烯/聚己内酯实验组通过Akt-eNOS-血管内皮生长因子信号通路促进血管生成,从而修复受损的坐骨神经,验证了氧化石墨烯的成血管作用。由于具有优异的机械性能、较大的比表面积和出色的导热性能,氧化石墨烯还被用作药物输送的载体。HUANG等[60]用β环糊精功能化修饰的氧化石墨烯负载一氧化氮供体药物BNN6(一种N-亚硝基化合物),在近红外光照射下,氧化石墨烯良好的光热性能促使BNN6分解并释放一氧化氮,通过一氧化氮-环磷鸟苷信号通路(NO-cGMP)促进血管生成,从而加速了伤口的愈合。碳纳米管也被用于成血管治疗。MASOTTI等[61]将多胺包裹的碳纳米管用作小分子核糖核酸的递送系统,通过靶向内皮细胞来调节血管生成。研究证明,巨噬细胞暴露于多壁碳纳米管后,可分泌与组织修复相关的分子,如基质金属蛋白酶9和血管内皮生长因子,从而促进血管生成[62]。具有成血管作用的金属元素和纳米材料,见表4。"
| [1] CARMELIET P, JAIN RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298-307. [2] GAHARWAR AK, SINGH I, KHADEMHOSSEINI A. Engineered biomaterials for in situ tissue regeneration. Nat Rev Mater. 2020;5(9):686-705. [3] CAPORARELLO N, D’ANGELI F, CAMBRIA MT, et al. Pericytes in microvessels: From “Mural” function to brain and retina regeneration. Int J Mol Sci. 2019; 20(24):6351. [4] WON JE, LEE YS, PARK JH, et al. Hierarchical microchanneled scaffolds modulate multiple tissue-regenerative processes of immune-responses, angiogenesis, and stem cell homing. Biomaterials. 2020;227:119548. [5] JALALI S, TAFAZZOLI-SHADPOUR M, HAGHIGHIPOUR N, et al. Regulation of endothelial cell adherence and elastic modulus by substrate stiffness. Cell Commun Adhes. 2015;22(2-6):79-89. [6] KRETSCHMER M, RüDIGER D, ZAHLER S. Mechanical aspects of angiogenesis. Cancers (Basel). 2021;13(19):4987. [7] JANNATBABAEI A, TAFAZZOLI-SHADPOUR M, SEYEDJAFARI E. Effects of substrate mechanics on angiogenic capacity and nitric oxide release in human endothelial cells. Ann N Y Acad Sci. 2020;1470(1):31-43. [8] WANG Y, ZHANG X, WANG W, et al. Integrin αVβ5/Akt/Sp1 pathway participates in matrix stiffness-mediated effects on VEGFR2 upregulation in vascular endothelial cells. Am J Cancer Res. 2020;10(8):2635-2648. [9] HUSAIN A, KHADKA A, EHRLICHER A, et al. Substrate stiffening promotes VEGF-A functions via the PI3K/Akt/mTOR pathway. Biochem Biophys Res Commun. 2022;586:27-33. [10] SAWANE M, OGURA Y, NAKAMURA A, et al. Blood vessels sense dermal stiffness via a novel mechanotransducer, APJ. Angiogenesis. 2022;25(2):151-154. [11] MASON BN, STARCHENKO A, WILLIAMS RM, et al. Tuning three-dimensional collagen matrix stiffness independently of collagen concentration modulates endothelial cell behavior. Acta Biomater. 2013;9(1):4635-4644. [12] GUO Y, MEI F, HUANG Y, et al. Matrix stiffness modulates tip cell formation through the p-PXN-Rac1-YAP signaling axis. Bioact Mater. 2022;7:364-376. [13] BETTINGER CJ, LANGER R, BORENSTEIN JT. Engineering substrate topography at the micro- and nanoscale to control cell function. Angew Chem Int Ed Engl. 2009;48(30):5406-5415. [14] MA C, KUZMA ML, BAI X, et al. Biomaterial-based metabolic regulation in regenerative engineering. Adv Sci. 2019;6(19):1900819. [15] CHUNG TW, LIU DZ, WANG SY, et al. Enhancement of the growth of human endothelial cells by surface roughness at nanometer scale. Biomaterials. 2003;24(25):4655-4661. [16] LIU M, WANG D, GU S, et al. Micro/nano materials regulate cell morphology and intercellular communication by extracellular vesicles. Acta Biomater. 2021;124:130-138. [17] MASCHARAK S, BENITEZ PL, PROCTOR AC, et al. YAP-dependent mechanotransduction is required for proliferation and migration on native-like substrate topography. Biomaterials. 2017;115:155-166. [18] TANG Z, WANG X, YANG J, et al. Microconvex dot-featured silk fibroin films for promoting human umbilical vein endothelial cell angiogenesis via enhancing the expression of bFGF and VEGF. ACS Biomater Sci Eng. 2021; 7(6):2420-2429. [19] YANG Y, LIN Y, ZHANG Z, et al. Micro/nano-net guides M2-pattern macrophage cytoskeleton distribution via Src-ROCK signalling for enhanced angiogenesis. Biomater Sci. 2021;9(9):3334-3347. [20] YANG C, ZHAO C, WANG X, et al. Stimulation of osteogenesis and angiogenesis by micro/nano hierarchical hydroxyapatite via macrophage immunomodulation. Nanoscale. 2019;11(38):17699-17708. [21] NI S, ZHAI D, HUAN Z, et al. Nanosized concave pit/convex dot microarray for immunomodulatory osteogenesis and angiogenesis. Nanoscale. 2020; 12(31):16474-16488. [22] JIN S, YANG R, CHU C, et al. Topological structure of electrospun membrane regulates immune response, angiogenesis and bone regeneration. Acta Biomater. 2021;129:148-158. [23] TIAN T, ZHANG T, LIN Y, et al. Vascularization in craniofacial bone tissue engineering. J Dent Res. 2018;97(9):969-976. [24] GRIFFON DJ, SEDIGHI MR, SCHAEFFER DV, et al. Chitosan scaffolds: interconnective pore size and cartilage engineering. Acta Biomater. 2006; 2(3):313-320. [25] CHOI SW, ZHANG Y, MACEWAN MR, et al. Neovascularization in biodegradable inverse opal scaffolds with uniform and precisely controlled pore sizes. Adv Healthc Mater. 2013;2(1):145-154. [26] BAI F, WANG Z, LU J, et al. The correlation between the internal structure and vascularization of controllable porous bioceramic materials in vivo: a quantitative study. Tissue Eng Part A. 2010;16(12):3791-3803. [27] YIN Y, HE XT, WANG J, et al. Pore size-mediated macrophage M1-to-M2 transition influences new vessel formation within the compartment of a scaffold. Appl Mater Today. 2020;18:100466. [28] WANG WY, KENT RN 3RD, HUANG SA, et al. Direct comparison of angiogenesis in natural and synthetic biomaterials reveals that matrix porosity regulates endothelial cell invasion speed and sprout diameter. Acta Biomater. 2021;135:260-273. [29] JIA G, HUANG H, NIU J, et al. Exploring the interconnectivity of biomimetic hierarchical porous Mg scaffolds for bone tissue engineering: Effects of pore size distribution on mechanical properties, degradation behavior and cell migration ability. J Magnes Alloy. 2021;9(6):1954-1966. [30] GUPTE MJ, SWANSON WB, HU J, et al. Pore size directs bone marrow stromal cell fate and tissue regeneration in nanofibrous macroporous scaffolds by mediating vascularization. Acta Biomater. 2018;82:1-11. [31] ALI D, SEN S. Finite element analysis of mechanical behavior, permeability and fluid induced wall shear stress of high porosity scaffolds with gyroid and lattice-based architectures. J Mech Behav Biomed Mater. 2017;75:262-270. [32] MEHDIZADEH H, BAYRAK ES, LU C, et al. Agent-based modeling of porous scaffold degradation and vascularization: optimal scaffold design based on architecture and degradation dynamics. Acta Biomater. 2015;27:167-178. [33] WANG S, HASHEMI S, STRATTON S, et al. The effect of physical cues of biomaterial scaffolds on stem cell behavior. Adv Healthc Mater. 2021;10(3): e2001244. [34] LIU J, LONG H, ZEUSCHNER D, et al. Synthetic extracellular matrices with tailored adhesiveness and degradability support lumen formation during angiogenic sprouting. Nat Commun. 2021;12(1):3402. [35] TRAPPMANN B, BAKER BM, POLACHECK WJ, et al. Matrix degradability controls multicellularity of 3D cell migration. Nat Commun. 2017;8(1):371. [36] 贺利贞.功能化介孔二氧化硅纳米载药体系在肿瘤诊疗中的应用与机制研究[D].广州:暨南大学,2020. [37] GUERRERO PA, MCCARTY JH. Integrins in vascular development and pathology. Adv Pharmacol. 2018;81:129-153. [38] MELCHIORRI AJ, HIBINO N, YI T, et al. Contrasting biofunctionalization strategies for the enhanced endothelialization of biodegradable vascular grafts. Biomacromolecules. 2015;16(2):437-446. [39] MOULISOVá V, GONZALEZ-GARCíA C, CANTINI M, et al. Engineered microenvironments for synergistic VEGF-Integrin signalling during vascularization. Biomaterials. 2017;126:61-74. [40] PARK TY, MAENG SW, JEON EY, et al. Adhesive protein-based angiogenesis-mimicking spatiotemporal sequential release of angiogenic factors for functional regenerative medicine. Biomaterials. 2021;272:120774. [41] BAI Y, BAI L, ZHOU J, et al. Sequential delivery of VEGF, FGF-2 and PDGF from the polymeric system enhance HUVECs angiogenesis in vitro and CAM angiogenesis. Cell Immunol. 2018;323:19-32. [42] TEIXEIRA SP, DOMINGUES RM, SHEVCHUK M, et al. Biomaterials for sequestration of growth factors and modulation of cell behavior. Adv Funct Mater. 2020;30(44):1909011 [43] WEAVER JD, HEADEN DM, AQUART J, et al. Vasculogenic hydrogel enhances islet survival, engraftment, and function in leading extrahepatic sites. Sci Adv. 2017;3(6):e1700184. [44] MARTINO MM, BRIQUEZ PS, Güç E, et al. Growth factors engineered for super-affinity to the extracellular matrix enhance tissue healing. Science. 2014;343(6173):885-888. [45] QU M, JIANG X, ZHOU X, et al. Stimuli-responsive delivery of growth factors for tissue engineering. Adv Healthc Mater. 2020;9(7):e1901714. [46] JOSHI RV, NELSON CE, POOLE KM, et al. Dual pH- and temperature-responsive microparticles for protein delivery to ischemic tissues. Acta Biomater. 2013;9(5):6526-6534. [47] STEJSKALOVá A, OLIVA N, ENGLAND FJ, et al. Biologically inspired, cell-selective release of aptamer-trapped growth factors by traction forces. Adv Mater. 2019;31(7):e1806380. [48] BARUI AK, NETHI SK, HAQUE S, et al. Recent development of metal nanoparticles for angiogenesis study and their therapeutic applications. ACS Appl Bio Mater. 2019;2(12):5492-5511. [49] 李俊刚.3D打印掺钴生物陶瓷支架介导成骨-成血管偶联促进骨修复的作用研究[D].福州:福建医科大学,2021. [50] 张竞心,刘林枫,张士文,等.镁离子促进骨再生的分子机制[J].中国组织工程研究,2022,26(33):5384-5392. [51] AUGUSTINE R, DALVI YB, YADU NATH VK, et al. Yttrium oxide nanoparticle loaded scaffolds with enhanced cell adhesion and vascularization for tissue engineering applications. Mater Sci Eng C Mater Biol Appl. 2019; 103:109801. [52] AUGUSTINE R, HASAN A, PATAN NK, et al. Titanium nanorods loaded pcl meshes with enhanced blood vessel formation and cell migration for wound dressing applications. Macromol Biosci. 2019;19(7):e1900058. [53] 吴羽翀,彭旭,余喜讯.掺铕聚磷酸钙骨组织工程支架可促成骨,血管生成及抗无菌性松动[J].中国组织工程研究,2022,26(28):4458-4465. [55] NOSRATI H, ARAMIDEH KHOUY R, NOSRATI A, et al. Nanocomposite scaffolds for accelerating chronic wound healing by enhancing angiogenesis. J Nanobiotechnol. 2021;19(1):1-21. [55] KARGOZAR S, BAINO F, HAMZEHLOU S, et al. Nanotechnology for angiogenesis: opportunities and challenges. Chem Soc Rev. 2020;49(14): 5008-5057. [56] LAU P, BIDIN N, ISLAM S, et al. Influence of gold nanoparticles on wound healing treatment in rat model: Photobiomodulation therapy. Lasers Surg Med. 2017;49(4):380-386. [57] CHEN Y, WU Y, GAO J, et al. Transdermal vascular endothelial growth factor delivery with surface engineered gold nanoparticles. ACS Appl Mater Interfaces. 2017;9(6):5173-5180. [58] MUKHERJEE S, SRIRAM P, BARUI AK, et al. Graphene oxides show angiogenic properties. Adv Healthc Mater. 2015;4(11):1722-1732. [59] QIAN Y, SONG J, ZHAO X, et al. 3D Fabrication with integration molding of a graphene oxide/polycaprolactone nanoscaffold for neurite regeneration and angiogenesis. Adv Sci (Weinh). 2018;5(4):1700499. [60] HUANG S, LIU H, LIAO K, et al. Functionalized GO nanovehicles with nitric oxide release and photothermal activity-based hydrogels for bacteria-infected wound healing. ACS Appl Mater Interfaces. 2020;12(26): 28952-28964. [61] MASOTTI A, MILLER MR, CELLUZZI A, et al. Regulation of angiogenesis through the efficient delivery of microRNAs into endothelial cells using polyamine-coated carbon nanotubes. Nanomed. 2016;12(6):1511-1522. [62] MENG J, LI X, WANG C, et al. Carbon nanotubes activate macrophages into a M1/M2 mixed status: recruiting naïve macrophages and supporting angiogenesis. ACS Appl Mater Interfaces. 2015;7(5):3180-3188. |
| [1] | Sun Kexin, Zeng Jinshi, Li Jia, Jiang Haiyue, Liu Xia. Mechanical stimulation enhances matrix formation of three-dimensional bioprinted cartilage constructs [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(在线): 1-7. |
| [2] | Xu Xingxing, Wen Chaoju, Meng Maohua, Wang Qinying, Chen Jingqiao, Dong Qiang. Carbon nanomaterials in oral implant [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7): 1062-1070. |
| [3] | Yang Yitian, Wang Lu, Yao Wei, Zhao Bin. Application of the interaction between biological scaffolds and macrophages in bone regeneration [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7): 1071-1079. |
| [4] | Li Cheng, Zheng Guoshuang, Kuai Xiandong, Yu Weiting. Alginate scaffold in articular cartilage repair [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7): 1080-1088. |
| [5] | Shi Yehong, Wang Cheng, Chen Shijiu. Early thrombosis and prevention of small-diameter blood vessel prosthesis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7): 1110-1116. |
| [6] | Tang Haotian, Liao Rongdong, Tian Jing. Application and design of piezoelectric materials for bone defect repair [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7): 1117-1125. |
| [7] | Xu Yan, Li Ping, Lai Chunhua, Zhu Peijun, Yang Shuo, Xu Shulan. Piezoelectric materials for vascularized bone regeneration [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7): 1126-1132. |
| [8] | Li Xinyue, Li Xiheng, Mao Tianjiao, Tang Liang, Li Jiang. Three-dimensional culture affects morphology, activity and osteogenic differentiation of human periodontal ligament stem cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 846-852. |
| [9] | Li Xiaoyin, Yang Xiaoqing, Chen Shulian, Li Zhengchao, Wang Ziqi, Song Zhen, Zhu Daren, Chen Xuyi. Collagen/silk fibroin scaffold combined with neural stem cells in the treatment of traumatic spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 890-896. |
| [10] | Yuan Bo, Xie Lide, Fu Xiumei. Schwann cell-derived exosomes promote the repair and regeneration of injured peripheral nerves [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 935-940. |
| [11] | Qin Yuxing, Ren Qiangui, Li Zilong, Quan Jiaxing, Shen Peifeng, Sun Tao, Wang Haoyu. Action mechanism and prospect of bone microvascular endothelial cells for treating femoral head necrosis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 955-961. |
| [12] | Xiong Bohan, Yu Yang, Lu Xiaojun, Wang Xu, Yang Tengyun, Zhang Yaozhang, Liao Xinyu, Zhou Xiaoxiang, He Lu, Li Yanlin. Research progress in promoting tendon to bone healing during anterior cruciate ligament reconstruction [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(5): 779-786. |
| [13] | Zhang Min, Zhang Xiaoming, Liu Tongbin. Application potential of naringin in bone tissue regeneration [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(5): 787-792. |
| [14] | Zong Mingrui, Liu Haiyan, Li Bing, Wu Xiuping. Application of carboxymethyl chitosan in tissue engineering of stomatology [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(3): 447-452. |
| [15] | Zhang Lichen, Chen Liang, Gu Yong. Inorganic ion bionic periosteum regulates immune microenvironment to promote bone repair [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(3): 346-353. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||