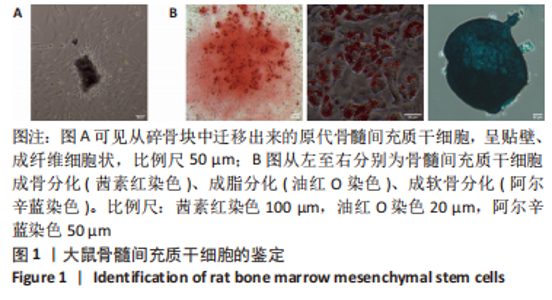
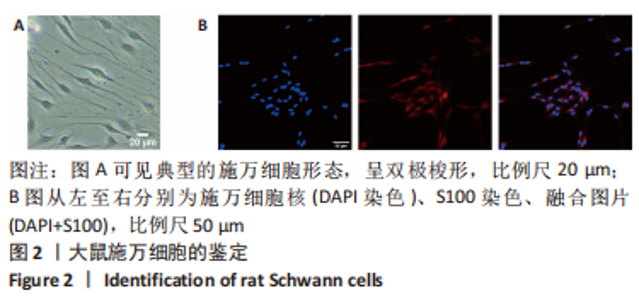
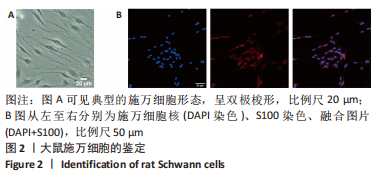
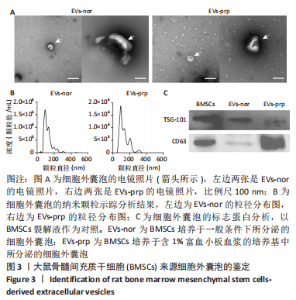
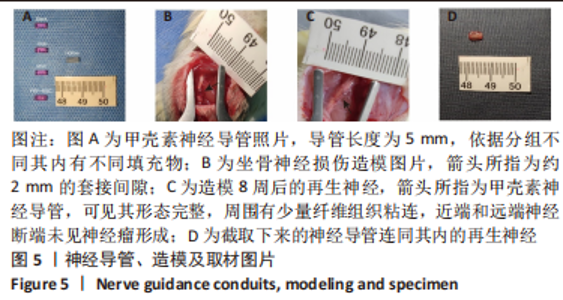
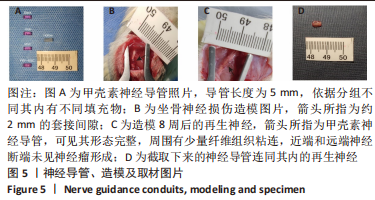
Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (7): 985-992.doi: 10.12307/2024.109
Combination of 1% platelet-rich plasma and bone marrow mesenchymal stem cells improves the recovery of peripheral nerve injury
Feng Ruiqin1, 2, 3, Han Na1, 2, 3, Zhang Meng1, 2, 3, Gu Xinyi1, 2, 3, Zhang Fengshi1, 2, 3
- 1Department of Orthopedics and Trauma, Peking University People’s Hospital, Beijing 100044, China; 2Key Laboratory of Trauma and Neural Regeneration, Ministry of Education, Beijing 100044, China; 3National Center for Trauma Medicine, Beijing 101100, China
-
Received:2023-02-01Accepted:2023-03-09Online:2024-03-08Published:2023-07-15 -
Contact:Han Na, MD, Researcher, Department of Orthopedics and Trauma, Peking University People’s Hospital, Beijing 100044, China; Key Laboratory of Trauma and Neural Regeneration, Ministry of Education, Beijing 100044, China; National Center for Trauma Medicine, Beijing 101100, China -
About author:Feng Ruiqin, Master candidate, Physician, Department of Orthopedics and Trauma, Peking University People’s Hospital, Beijing 100044, China; Key Laboratory of Trauma and Neural Regeneration, Ministry of Education, Beijing 100044, China; National Center for Trauma Medicine, Beijing 101100, China -
Supported by:Natural Science Foundation of Beijing, No. 7222198 (to HN)
CLC Number:
Cite this article
Feng Ruiqin, Han Na, Zhang Meng, Gu Xinyi, Zhang Fengshi. Combination of 1% platelet-rich plasma and bone marrow mesenchymal stem cells improves the recovery of peripheral nerve injury[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 985-992.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
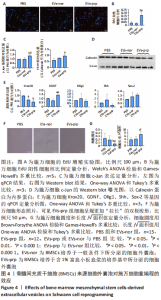
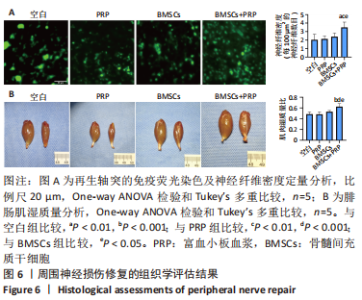
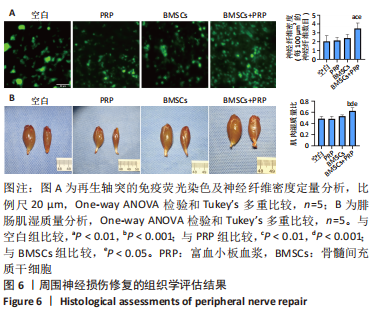
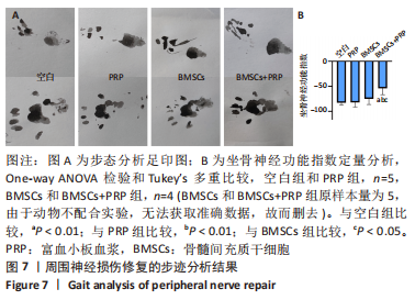
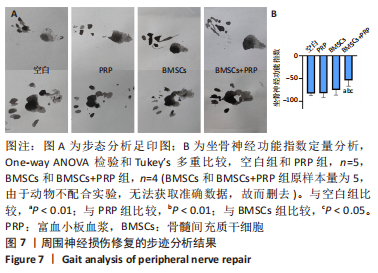
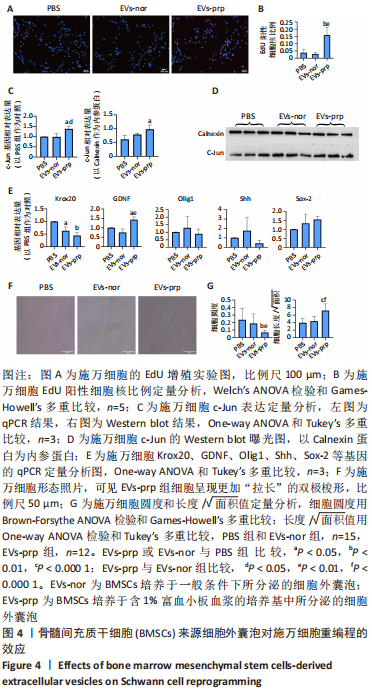
2.3 EVs-prp具有更强的施万细胞重编程刺激功能 施万细胞重编程的特点主要包括:①细胞增殖能力增强;②c-Jun表达上调,c-Jun是驱动施万细胞重编程最关键的转录因子[30-31];③髓鞘化相关基因表达下调;④某些特征性基因表达上调,如胶质细胞源性神经营养因子(glial cell line-derived neurotrophic factor,GDNF)、Olig1、Shh、Sox-2等;⑤获得极度拉长的双极梭形形态以便形成再生轨道,有助于后续引导轴突再生[20]。 对这些重编程特点的分析可以了解施万细胞的重编程状态。EdU细胞增殖实验结果见图4A,B。EVs-prp组EdU阳性细胞核比例显著高于PBS组和EVs-nor组(P < 0.01),且EVs-nor和PBS组之间没有显著差异,表明EVs-prp能够显著刺激施万细胞增殖。转录因子c-Jun的表达分析结果显示,EVs-prp能够刺激施万细胞 c-Jun表达,其基因和蛋白表达量显著高于PBS组(P < 0.05),同时基因表达量显著高于EVs-nor组(P < 0.05),见图4C,D。髓鞘化相关基因Krox20的 qPCR检测结果显示,与PBS组相比,EVs-nor和EVs-prp组Krox20表达均出现显著下调(EVs-nor,P < 0.05;EVs-prp,P < 0.01),EVs-nor和EVs-prp组之间无显著性差异;GDNF基因表达在EVs-prp组中上调,显著高于PBS组和EVs-nor组(PBS,P < 0.05;EVs-nor,P < 0.01),PBS组和EVs-nor组之间没有显著差异,见图4E。最后,施万细胞形态分析结果显示,EVs-prp组细胞的圆度显著低于PBS和EVs-nor组(PBS,P < 0.01;EVs-nor,P < 0.01),且细胞长度/√面积值显著高于PBS和EVs-nor组(PBS,P < 0.000 1;EVs-nor,P < 0.000 1),同时这2个值在PBS组和EVs-nor组之间没有显著差异,见图4F,G。这表明EVs-prp组细胞形态显著伸长。"
| [1] LI R, LIU Z, PAN Y, et al. Peripheral nerve injuries treatment: a systematic review. Cell Biochem Biophys. 2014;68(3):449-454. [2] CARVALHO CR, REIS RL, OLIVEIRA JM. Fundamentals and Current Strategies for Peripheral Nerve Repair and Regeneration. Adv Exp Med Biol. 2020;1249:173-201. [3] VIJAYAVENKATARAMAN S. Nerve guide conduits for peripheral nerve injury repair: A review on design, materials and fabrication methods. Acta Biomater. 2020;106:54-69. [4] MEENA P, KAKKAR A, KUMAR M, et al. Advances and clinical challenges for translating nerve conduit technology from bench to bed side for peripheral nerve repair. Cell Tissue Res. 2021;383(2):617-644. [5] 沈君劼,林俊卿,索金龙,等.组织工程化神经导管治疗周围神经损伤[J].国际骨科学杂志,2021,42(6): 379-383. [6] 龚超,张玉强,王伟,等.细胞治疗周围神经损伤的作用及机制[J].中国组织工程研究,2022,26(13): 2114-2119. [7] HAN Y, YANG J, FANG J, et al. The secretion profile of mesenchymal stem cells and potential applications in treating human diseases. Signal Transduct Target Ther. 2022;7(1):92. [8] HOANG DM, PHAM PT, BACH TQ, et al. Stem cell-based therapy for human diseases. Signal Transduct Target Ther. 2022;7(1):272. [9] GALDERISI U, PELUSO G, DI BERNARDO G. Clinical Trials Based on Mesenchymal Stromal Cells are Exponentially Increasing: Where are We in Recent Years? Stem Cell Rev Rep. 2022;18(1):23-36. [10] YOUSEFI F, LAVI ARAB F, NIKKHAH K, et al. Novel approaches using mesenchymal stem cells for curing peripheral nerve injuries. Life Sci. 2019;221:99-108. [11] CUI Y, YAO Y, ZHAO Y, et al. Functional collagen conduits combined with human mesenchymal stem cells promote regeneration after sciatic nerve transection in dogs. J Tissue Eng Regen Med. 2018;12(5):1285-1296. [12] RODRÍGUEZ-SÁNCHEZ DN, PINTO GBA, CARTAROZZI LP, et al. 3D-printed nerve guidance conduits multi-functionalized with canine multipotent mesenchymal stromal cells promote neuroregeneration after sciatic nerve injury in rats. Stem Cell Res Ther. 2021;12(1):303. [13] HERSANT B, SID-AHMED M, BRAUD L, et al. Platelet-Rich Plasma Improves the Wound Healing Potential of Mesenchymal Stem Cells through Paracrine and Metabolism Alterations. Stem Cells Int. 2019;2019:1234263. [14] LEVOUX J, PROLA A, LAFUSTE P, et al. Platelets facilitate the wound-healing capability of mesenchymal stem cells by mitochondrial transfer and metabolic reprogramming. Cell Metab. 2021;33(3):688-690. [15] FERREIRA JR, TEIXEIRA GQ, SANTOS SG, et al. Mesenchymal Stromal Cell Secretome: Influencing Therapeutic Potential by Cellular Pre-conditioning. Front Immunol. 2018;9:2837. [16] VAN NIEL G, CARTER DRF, CLAYTON A, et al. Challenges and directions in studying cell-cell communication by extracellular vesicles. Nat Rev Mol Cell Biol. 2022;23(5):369-382. [17] VAN NIEL G, D’ANGELO G, RAPOSO G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213-228. [18] MAO Q, NGUYEN PD, SHANTI RM, et al. Gingiva-Derived Mesenchymal Stem Cell-Extracellular Vesicles Activate Schwann Cell Repair Phenotype and Promote Nerve Regeneration. Tissue Eng Part A. 2019;25(11-12):887-900. [19] JESSEN KR, MIRSKY R. The repair Schwann cell and its function in regenerating nerves. J Physiol. 2016;594(13):3521-3531. [20] JESSEN KR, ARTHUR-FARRAJ P. Repair Schwann cell update: Adaptive reprogramming, EMT, and stemness in regenerating nerves. Glia. 2019;67(3):421-437. [21] NOCERA G, JACOB C. Mechanisms of Schwann cell plasticity involved in peripheral nerve repair after injury. Cell Mol Life Sci. 2020;77(20):3977-3989. [22] WANG Y, LIN J, CHEN J, et al. Biodegradable polyurethane-incorporating decellularized spinal cord matrix scaffolds enhance Schwann cell reprogramming to promote peripheral nerve repair. J Mater Chem B. 2023;11(10):2115-2128. [23] JACOBS FA, VAN DE VYVER M, FERRIS WF. Isolation and Characterization of Different Mesenchymal Stem Cell Populations from Rat Femur. Methods Mol Biol. 2019;1916:133-147. [24] 张学磊,罗干,于圣会,等.脱细胞神经联合骨髓间充质干细胞及富血小板凝胶治疗股神经损伤[J].中国组织工程研究,2022,26(1):27-32. [25] MAUREL P. Preparation of Neonatal Rat Schwann Cells and Embryonic Dorsal Root Ganglia Neurons for In Vitro Myelination Studies. Methods Mol Biol. 2018;1739:17-37. [26] LI H, LI B. PRP as a new approach to prevent infection: preparation and in vitro antimicrobial properties of PRP. J Vis Exp. 2013;(74):50351. [27] GORGUN C, CERESA D, LESAGE R, et al. Dissecting the effects of preconditioning with inflammatory cytokines and hypoxia on the angiogenic potential of mesenchymal stromal cell (MSC)-derived soluble proteins and extracellular vesicles (EVs). Biomaterials. 2021;269:120633. [28] FANG X, DENG J, ZHANG W, et al. Conductive conduit small gap tubulization for peripheral nerve repair. RSC Adv. 2020;10(28):16769-16775. [29] ZHANG P, HAN N, WANG T, et al. Biodegradable conduit small gap tubulization for peripheral nerve mutilation: a substitute for traditional epineurial neurorrhaphy. Int J Med Sci. 2013;10(2):171-175. [30] JESSEN KR, MIRSKY R. The Role of c-Jun and Autocrine Signaling Loops in the Control of Repair Schwann Cells and Regeneration. Front Cell Neurosci. 2022;15: 820216. [31] ARTHUR-FARRAJ PJ, LATOUCHE M, WILTON DK, et al. c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron. 2012;75(4):633-647. [32] SHANG F, YU Y, LIU S, et al. Advancing application of mesenchymal stem cell-based bone tissue regeneration. Bioact Mater. 2020;6(3):666-683. [33] LE H, XU W, ZHUANG X, et al. Mesenchymal stem cells for cartilage regeneration. J Tissue Eng. 2020;11:2041731420943839. [34] COSTA-ALMEIDA R, CALEJO I, GOMES ME. Mesenchymal Stem Cells Empowering Tendon Regenerative Therapies. Int J Mol Sci. 2019;20(12):3002. [35] RAO F, ZHANG D, FANG T, et al. Exosomes from Human Gingiva-Derived Mesenchymal Stem Cells Combined with Biodegradable Chitin Conduits Promote Rat Sciatic Nerve Regeneration. Stem Cells Int. 2019;2019:2546367. [36] ZHANG W, FANG XX, LI QC, et al. Reduced graphene oxide-embedded nerve conduits loaded with bone marrow mesenchymal stem cell-derived extracellular vesicles promote peripheral nerve regeneration. Neural Regen Res. 2023;18(1):200-206. [37] LI C, LIU SY, ZHANG M, et al. Sustained release of exosomes loaded into polydopamine-modified chitin conduits promotes peripheral nerve regeneration in rats. Neural Regen Res. 2022;17(9):2050-2057. [38] BROSIUS LUTZ A, LUCAS TA, CARSON GA, et al. An RNA-sequencing transcriptome of the rodent Schwann cell response to peripheral nerve injury. J Neuroinflammation. 2022;19(1):105. [39] LIU JH, TANG Q, LIU XX, et al. Analysis of transcriptome sequencing of sciatic nerves in Sprague-Dawley rats of different ages. Neural Regen Res. 2018;13(12):2182-2190. [40] WELLEFORD AS, QUINTERO JE, SEBLANI NE, et al. RNA Sequencing of Human Peripheral Nerve in Response to Injury: Distinctive Analysis of the Nerve Repair Pathways. Cell Transplant. 2020;29:963689720926157. [41] HUANG J, ZHANG G, LI S, et al. Endothelial cell-derived exosomes boost and maintain repair-related phenotypes of Schwann cells via miR199-5p to promote nerve regeneration. J Nanobiotechnology. 2023;21(1):10. [42] MA Y, ZHOU D, ZHANG H, et al. Human Umbilical Cord Mesenchymal Stem Cell-Derived Extracellular Vesicles Promote the Proliferation of Schwann Cells by Regulating the PI3K/AKT Signaling Pathway via Transferring miR-21. Stem Cells Int. 2021;2021:1496101. [43] YANG J, WANG B, WANG Y, et al. Exosomes Derived from Adipose Mesenchymal Stem Cells Carrying miRNA-22-3p Promote Schwann Cells Proliferation and Migration through Downregulation of PTEN. Dis Markers. 2022;2022:7071877. [44] REGMI S, RAUT PK, PATHAK S, et al. Enhanced viability and function of mesenchymal stromal cell spheroids is mediated via autophagy induction. Autophagy. 2021;17(10):2991-3010. [45] BALDARI S, DI ROCCO G, PICCOLI M, et al. Challenges and Strategies for Improving the Regenerative Effects of Mesenchymal Stromal Cell-Based Therapies. Int J Mol Sci. 2017;18(10):2087. [46] WU J, PIAO Y, LIU Q, et al. Platelet-rich plasma-derived extracellular vesicles: A superior alternative in regenerative medicine? Cell Prolif. 2021;54(12):e13123. [47] 马婉茹,聂志扬,胡俊华,等.富血小板血浆的临床应用[J].临床输血与检验, 2021,23(6):806-811. |
| [1] | Qiu Xiaoyan, Li Bixin, Li Jingdi, Fan Chuiqin, Ma Lian, Wang Hongwu. Differentiation of insulin-producing cells from human umbilical cord mesenchymal stem cells infected by MAFA-PDX1 overexpressed lentivirus [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1000-1006. |
| [2] | Liu Qiwei, Zhang Junhui, Yang Yuan, Wang Jinjuan. Role and mechanism of umbilical cord mesenchymal stem cells on polycystic ovary syndrome [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1015-1020. |
| [3] | Liu Jianhong, Liao Shijie, Li Boxiang, Tang Shengping, Wei Zhendi, Ding Xiaofei. Extracellular vesicles carrying non-coding RNA regulate the activation of osteoclasts [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1076-1082. |
| [4] | Pan Xiaolong, Fan Feiyan, Ying Chunmiao, Liu Feixiang, Zhang Yunke. Effect and mechanism of traditional Chinese medicine on inhibiting the aging of mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1091-1098. |
| [5] | Liu Hanfeng, Wang Jingjing, Yu Yunsheng. Artificial exosomes in treatment of myocardial infarction: current status and prospects [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1118-1123. |
| [6] | Zhuge Xiaoxuan, Li Ce, Bao Guangjie, Kang Hong. Potential value of canonical and non-canonical roles of connexin 43 in disease treatment [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1130-1136. |
| [7] | Ma Shuwei, He Sheng, Han Bing, Zhang Liaoyun. Exosomes derived from mesenchymal stem cells in treatment of animals with acute liver failure: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1137-1142. |
| [8] | Zhang Kefan, Shi Hui. Research status and application prospect of cytokine therapy for osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(6): 961-967. |
| [9] | Wei Yuanxun, Chen Feng, Lin Zonghan, Zhang Chi, Pan Chengzhen, Wei Zongbo. The mechanism of Notch signaling pathway in osteoporosis and its prevention and treatment with traditional Chinese medicine [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 587-593. |
| [10] | Lin Feng, Cheng Ling, Gao Yong, Zhou Jianye, Shang Qingqing. Hyaluronic acid hydrogel-encapsulated bone marrow mesenchymal stem cells promote cardiac function in myocardial infarction rats (III) [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(3): 355-359. |
| [11] | Bi Yujie, Ma Dujun, Peng Liping, Zhou Ziqiong, Zhao Jing, Zhu Houjun, Zhong Qiuhui, Yang Yuxin. Strategy and significance of Chinese medicine combined with medical hydrogel for disease treatment [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(3): 419-425. |
| [12] | Long Yi, Yang Jiaming, Ye Hua, Zhong Yanbiao, Wang Maoyuan. Extracellular vesicles in sarcopenic obesity: roles and mechanisms [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(2): 315-320. |
| [13] | Li Chengming, Xue Dongling, Yang Xinyu, Xiao Chi, Cui Daping. Mechanism of Chinese medicine for promoting blood circulation and removing blood stasis combined with platelet-rich plasma to improve steroid-induced necrosis of the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(2): 288-294. |
| [14] | Fan Yongjing, Wang Shu, Jin Wulong. Characteristics, advantages and application of osteogenic differentiation of jaw bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(1): 100-106. |
| [15] | Huang Yongbin, Wang Tao, Lou Yuanyi, Pang Jingqun, Chen Guanghua. Application prospect of mesenchymal stem cells in promoting muscle tissue repair [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(1): 107-112. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||