Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (7): 1130-1136.doi: 10.12307/2023.905
Previous Articles Next Articles
Potential value of canonical and non-canonical roles of connexin 43 in disease treatment
Zhuge Xiaoxuan1, Li Ce2, Bao Guangjie1, Kang Hong2
- 1Key Lab of Stomatology of State Ethnic Affairs Commission, Northwest Minzu University, Lanzhou 730030, Gansu Province, China; 2School of Stomatology, Lanzhou University, Lanzhou 730000, Gansu Province, China
-
Received:2022-10-17Accepted:2023-02-08Online:2024-03-08Published:2023-07-17 -
Contact:Bao Guangjie, Master, Professor, Master’s supervisor, Key Lab of Stomatology of State Ethnic Affairs Commission, Northwest Minzu University, Lanzhou 730030, Gansu Province, China Kang Hong, MD, PhD, Professor, Master’s supervisor, School of Stomatology, Lanzhou University, Lanzhou 730000, Gansu Province, China -
About author:Zhuge Xiaoxuan, Key Lab of Stomatology of State Ethnic Affairs Commission, Northwest Minzu University, Lanzhou 730030, Gansu Province, China Li Ce, Master candidate, School of Stomatology, Lanzhou University, Lanzhou 730000, Gansu Province, China -
Supported by:National Natural Science Foundation of China, No. 81660189 (to BGJ); Natural Science Foundation of Gansu Province, No. 21JR7RA161 (to BGJ); Basic Research Funds of Central Universities of Northwest Minzu University, No. 31920220013 (to BGJ); National Innovation and Entrepreneurship Training Program for College Students, No. 202110742055 (to ZGXX)
CLC Number:
Cite this article
Zhuge Xiaoxuan, Li Ce, Bao Guangjie, Kang Hong. Potential value of canonical and non-canonical roles of connexin 43 in disease treatment[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1130-1136.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
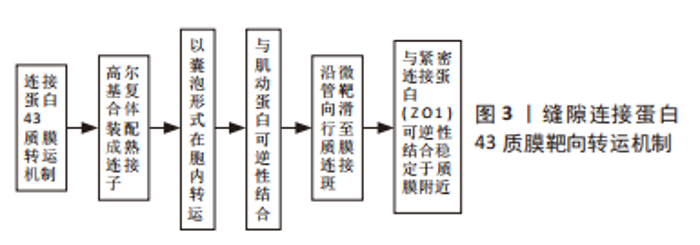
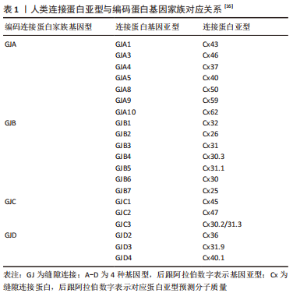
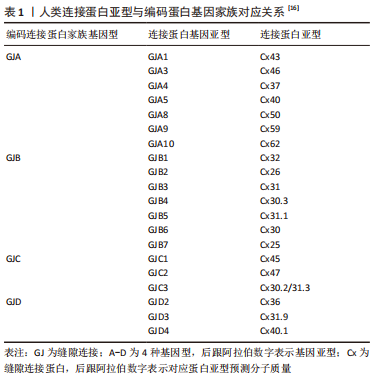
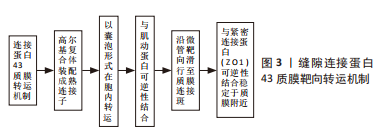
在人类组织中,研究最多、分布最广泛的连接蛋白亚型是缝隙连接蛋白43[8]。全长缝隙连接蛋白43为长度382个氨基酸(aa)的跨膜蛋白,由4个保守的α-螺旋跨膜结构域、2个胞外环、一个胞质环和胞质氨基(NT)端及羧基端(CT)结构域组成。NT端较短(13个氨基酸),CT端较长(150个氨基酸)。CT端多个区域被用作构建Cx拟肽的肽序列,如αCT1、Gap19、Gap26和Gap27,常作为体内外实验探究缝隙连接蛋白43功能的工具[2]。当然,在选择性剪切下,仍可形成多样性截短缝隙连接蛋白43片段。缝隙连接蛋白43与目前发现的大多连接蛋白都遵循着细胞核转录、内质网翻译修饰的常规胞内合成途径。SMYTH等[9]研究说明缝隙连接蛋白43以囊泡形式由粗面内质网向高尔基复合体顺式面转运的过程中,多数游离的单体连接蛋白已经开始寡聚形成六聚体,然后经顺式面移至反式面,成为具有通道形成功能的成熟连接子后退出高尔基复合体。由此可见,在缝隙连接蛋白43合成早期便已经初具半通道样结构,而不是在转运至质膜时形成,这也为多样形式缝隙连接蛋白43在亚细胞定位的转运机制提供了物质基础。因此开展更多基于缝隙连接蛋白43在高尔基复合体至质膜及细胞器膜等部位复杂转运机制的研究有可能助力种子细胞转化机制等组织工程领域的研究。 缝隙连接蛋白43的半衰期很短,仅为1-3 h,因此,连接蛋白的合成效率和定位于不同亚结构域的转运速率,对于维持缝隙连接在细胞间偶联过程具有重要作用 [10-11]。虽然目前连接子高效靶向运输到特定亚结构的具体机制尚未获得统一共识,但EPIFANTSEVA等[12]对于缝隙连接蛋白43质膜转运机制的研究及总结也为其复杂靶向转运网络奠定了坚实基础,见图3。经过上述转运机制,部分连接子靶向运输到质膜附近连接斑处,经囊泡卸载后,与紧密连接蛋白ZO1可逆性结合,等待羧基末端磷酸化事件触发后,便与相邻细胞的功能性连接蛋白六聚体联合形成缝隙连接。然而并非所有合成的缝隙连接蛋白43六聚体都转运于质膜上完成缝隙连接对接,它也可通过半通道完成自分泌及旁分泌过程而发挥非经典作用。并且其丰富的亚细胞定位-线粒体内膜、细胞内外囊泡等也通过非经典作用广泛参与多种生理及病理过程。因此,推测在质膜、线粒体内膜等附近存在不同靶向结合亚结构域,该结构可以特异性锚定转运至此的缝隙连接蛋白43,使缝隙连接蛋白43呈现了丰富的胞内定位。总之,其转运机制与缝隙连接蛋白43行使完整性功能密切相关,需要更多充分的研究来对此问题进一步探讨。"
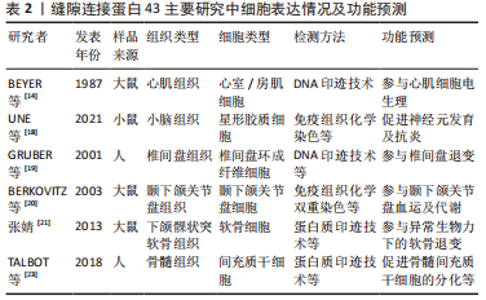
2.1.2 缝隙连接蛋白43的表达分布现状 作为一个复杂的具有相互作用的蛋白质网络组分的缝隙连接蛋白43,现有研究显示其在包括上皮细胞、内皮细胞、免疫细胞、器官型心脏培养物释放的细胞外囊泡和线粒体内膜等几乎每一种人体组织细胞甚至亚细胞结构中都普遍表达 [13],并且由于丰富的亚细胞定位,其可以细胞外囊泡等形式在全身血液循环中运动,广泛参与多种长距离物质转运等人体组织内生理病理活动,因此常与多种人类疾病密切联系。 缝隙连接蛋白43最早发现表达于心肌组织中,而后随着研究手段和方法的丰富与提升,其在人体组织细胞的广泛表达分布也逐渐明晰,但也正是由于其表达谱系广大,常难以系统地串联其各作用间的完整关系,因此学者多以临床疾病为着眼点,更多关注多发及高发疾病,如心血管疾病、神经系统疾病、骨关节退行性变和炎性疾病等。现存研究主要集中在心肌细胞、神经细胞、软骨细胞和骨细胞等[14-24],部分研究成果见表2。"
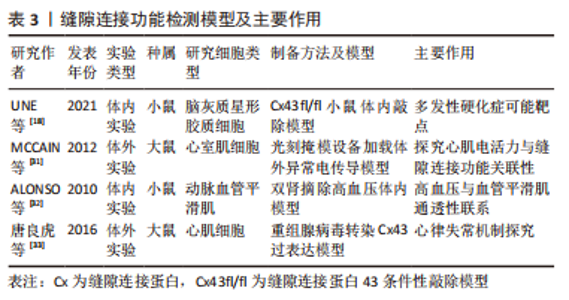
2.2 缝隙连接蛋白43的经典作用及其与疾病的关系 部分缝隙连接蛋白43六聚体经胞内转运到达质膜后与相邻细胞对接直接构成缝隙连接通道,从而参与细胞间物质运输,这种通道作用在表达缝隙连接蛋白43的人体细胞中较为普遍,因此受到众多学者的关注,常称其为缝隙连接蛋白43的经典通道依赖性作用。它具有隔绝细胞外微环境、直接完成细胞间信号高效递送的优势[26],因此而产生的致病性也牵连 广泛[27]。 缝隙连接以2-4 nm的微小缝隙构建胞间通道,其仅允许1 000 Da以下的物质通行,包括氨基酸、葡萄糖、各种离子、ATP、二磷酸腺苷、环磷酸腺苷、三磷酸肌醇、谷胱甘肽和谷氨酸等。依据通行物质理化性质常将其生理功能分为2类:①通过Na+、K+、Ca2+等离子交换的门控通道,进行电、化学信号传导;②通过细胞间代谢产物和第二信使等分子耦合调控胞间生物学过程[28-30]。 在相关组织疾病病理过程背后,常呈现出缝隙连接功能失衡受损现象,如在第一部分表达谱中发现的缺血性心肌病[15]、退行性椎间盘疾病等皆与缝隙连接异常表达相关[19],提示了缝隙连接通道参与疾病病理机制的高度可能性。而为了高效地探索其功能受损后病理状态下的分子调控机制,许多学者开展了研究,提供了丰富的体内外缝隙连接蛋白43相关疾病模型,如大鼠体外心室肌细胞电传导异常模型[31]、小鼠体内高血压模型[32],大鼠体外重组腺病毒缝隙连接蛋白43过表达模型[33]、小鼠脑灰质星形胶质细胞缝隙连接蛋白43敲除模型等[18,31-33],为解决缝隙连接蛋白43构成的缝隙连接结构功能失衡导致的心血管疾病、神经疾病等提供了丰富的研究手段,见表3。"

此外,缝隙连接结构在传递信息的同时,还具有细胞与细胞间的黏附特性[34-35],故也参与维持内皮细胞、上皮细胞等屏障保护作用。在病理损伤状态下,细胞屏障功能受损,而缝隙连接蛋白43构成的缝隙连接常表现为异常开放状态,提示缝隙连接黏附性也是其结构完整性的重要基础,这从ELIAS等[36]的实验中可以得到较为准确的佐证,他们在研究神经元径向迁移缺陷的重大问题中,发现去除缝隙连接通道离子转导及信号偶联干扰后,引入突变体形成了无黏附性的缝隙连接结构,其结果却未能恢复神经元径向迁移的表型。这启示通道结构本身特性对于缝隙连接通道行使功能必不可少,也开辟了以完整黏附性在高丰度表达缝隙连接通道相关疾病具体机制探索的新方法。赵希伟等[37]在体外急性肺损伤模型研究中得出了肺泡功能破坏与缝隙连接通讯负向相关的结论,但可惜的是作者未检测缝隙连接通道的黏附性所发生的变化,因此还不能完全判定缝隙连接通讯的过度表达是否具有其功能性,亦或是通道结构受损的标志。而另一项非病理损伤状态下的研究表明,添加缝隙连接阻断剂18β-甘草次酸和油酰胺后显著降低了血脑屏障内皮细胞的屏障功能[38],其改进之处在于结合了缝隙连接蛋白43与紧密连接蛋白ZO1共定位,干预后缝隙连接蛋白43与紧密连接蛋白ZO1表达并不受影响,但以缝隙连接通道为结构基础的屏障功能却显著性下降。故而间接印证了缝隙连接通道黏附性的重要结构基础。当然,这是否还要考虑不同形式的缝隙连接蛋白43共同参与因素,则需另行研究探讨。总的来说,缝隙连接通道的经典作用不是独立运作,而与多种连接方式密切相关,需要综合考虑其完整性,才能客观地为相关疾病诊断、治疗提供科学依据。"

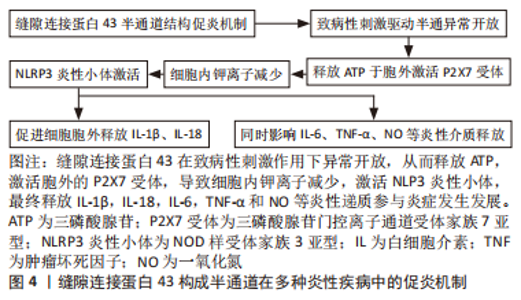
2.3 缝隙连接蛋白43的非经典作用及其与疾病的关系 2.3.1 缝隙连接蛋白43构成的细胞膜半通道作用 部分定位于质膜的缝隙连接蛋白43六聚体通过羧基末端多个位点的磷酸化后被激活,从而与相邻细胞对接嵌合成缝隙连接通道,但未触发对接的连接子本身也可以半通道形式通过自分泌、旁分泌途径完成细胞自身生理活动以及细胞与细胞外基质间的物质交换[39],并参与多种疾病病理过程。GUTSTEIN等[40]在癌细胞迁移信号通路中即验证了半通道自分泌的功能,XING等[41]则系统性阐述了在神经退行性疾病中半通道输送营养物质和能量至细胞外基质从而维持神经细胞活性的重要意义。但在此类疾病中缝隙连接蛋白43所构成的半通道结构是否稳定存在,还是只是缝隙连接通道结合前的瞬时效应,这决定了半通道所能发挥的效能大小。 炎症是一种复杂的免疫防御病理过程,其通过激活多种免疫细胞(T细胞、B细胞、单核细胞及中性粒细胞等)、黏附分子和炎症递质发挥双刃剑作用。免疫细胞由于自身特性不能与体内细胞建立稳定联系,故半通道常被认为是促使其游走迁移于全身组织和循环时交换信号的主要功能形式[42-44]。以上特征决定了缝隙连接蛋白43构成的半通道成为免疫细胞促炎过程的重要启动者,即在机体异常状态下参与调控包括神经退行性疾病、糖尿病、动脉粥样硬化等多种炎性疾病的病理机制[45-47],见图4。"
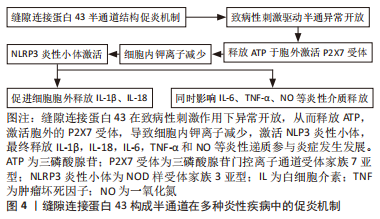
除此之外,NLRP3炎性小体激活后还可以通过pH变化、氧化应激和钙离子动员等非机械性刺激触发半通道活性[48]。这样正反馈式的循环使得炎症递质不断释放,从而造成机体最终的炎症状态。PRICE等[49]发现阻断构建人肾小管上皮细胞炎症模型中缝隙连接蛋白43构成的半通道后,可减轻早期肾小管损伤。MUGISHO等[50]则指出缝隙连接蛋白43构成的半通道参与糖尿病视网膜病变中视网膜的水肿、出血等病理过程。薛俊杰等[51]构建的小鼠骨关节炎模型中,缝隙连接蛋白43表达及半通道形成升高。以上研究较全面地佐证了缝隙连接蛋白43构成的半通道调控炎性疾病的重要发生发展环节,并且提示从上游机制看到预防或治愈此类炎性疾病的多重可能性。虽然包括上述研究的多种实验依据提示通过阻断剂(脂肪醇类的辛醇和庚醇、麻醉剂如氟烷、甘草酸衍生物的18α-甘草次酸及G毒毛旋花苷等[52])暂时抑制缝隙连接蛋白43半通道后,可以减轻水肿、抑制炎症、改善创伤修复和减少瘢痕形成[53],但单纯的通道抑制剂较难靶向抑制半通道结构,并且难以维持稳定且长久的疗效。总之,控制通道开放的靶点主要集中在羧基末端的氨基酸序列,因此模拟连接蛋白羧基末端尾部的肽链结构,开发设计靶向连接蛋白半通道抑制剂意义重大。已开发的模拟肽Gap19虽可相对靶向抑制缝隙连接蛋白43半通道开放[54],但是此类模拟肽常具有半衰期过短的缺憾,阻碍了研究的进一步发展和临床应用。因此,开发更具靶向潜力且性质稳定的半通道抑制剂对半通道相关疾病的诊疗具有重要价值。 2.3.2 缝隙连接蛋白43的细胞亚定位 缝隙连接蛋白43除了构成细胞膜上的缝隙连接通道及半通道之外,也被发现定位于其他细胞内外亚结构,如线粒体、细胞外囊泡等。这提示了缝隙连接蛋白43常以多种形式同时存在,其参与细胞生理活动与疾病病理过程时,所发挥协同抑或是拮抗效应,值得深入研究与思考。 缝隙连接蛋白43的线粒体定位:线粒体定位的缝隙连接蛋白43最早在心肌细胞中通过免疫金染色和透射电子显微镜技术观察到[55],随后,在多种细胞类型的线粒体中也检测到全长缝隙连接蛋白43,包括星形胶质细胞、人脐静脉内皮细胞、棕色脂肪组织中的脂肪细胞等[56-58]。以上组织细胞的共性表达显示了缝隙连接蛋白43线粒体定位也是缝隙连接蛋白43六聚体胞内转运网络所覆盖除质膜外的重要亚结构域之一,故思考六聚体转运机制在质膜与线粒体膜处是异曲同工还是各自独立,对于解开缝隙连接蛋白43多样亚定位特性具有重要意义。 这些线粒体定位的缝隙连接蛋白43六聚体经由上述的转运网络,在线粒体附近,由复杂的热休克蛋白途径调控,从线粒体外锚定缝隙连接蛋白43的亚结构域转入线粒体内膜上形成连接子样的通道结构,其作为线粒体膜通道被发现可以调节活性氧的产生、改善三磷酸腺苷水平、控制线粒体钾离子交换、维持线粒体呼吸代谢等[59]。除此之外,线粒体缝隙连接蛋白43在控制钙离子摄入与细胞损伤间关系仍处于研究阶段,但也得到了一些证据支持,如GADICHERLA等[60]通过缺血/再灌注模型证实了缝隙连接蛋白43与线粒体钙离子稳态的重要联系。但另有研究指出缝隙连接蛋白43存在于钙保持能力较差的线粒体亚群中[61],并且连接蛋白40耗竭模型也被发现参与线粒体钙离子稳态[62]。因此,不同连接蛋白亚型在线粒体定位的占比以及不同线粒体亚群间转运机制的差异都将影响线粒体功能的完整性,这提示可以通过多途径靶向阻断线粒体缝隙连接蛋白43,以探讨其造成相关疾病背后的多元络网。 构成线粒体内膜通道的缝隙连接蛋白43表达量随增龄变化呈下降趋势[63],通过构建衰老动物模型来研究这一特性,则显示出了线粒体呼吸代谢与能量摄入障碍[64]。这可能与线粒体缝隙连接蛋白43受到一些在衰老中普遍存在的有害翻译后修饰有关。值得注意的是,线粒体本身几乎不编码缝隙连接蛋白43基因,缝隙连接蛋白43通过上述途径嵌入线粒体内膜后,其动态效应与代谢途径是否如质膜缝隙连接蛋白43一样仍不清楚,可能需要拓宽缝隙连接蛋白43蛋白互作分子的研究进一步论证。 缝隙连接蛋白43 在线粒体转移中也可能具有关键作用,限制于胞内线粒体可以在生理和应激条件下进行细胞间转移,以维持内环境稳态或修复受损组织[65],而诱导缺乏线粒体缝隙连接蛋白43的细胞则会导致该过程受阻[66–68]。然而要注意的是,缝隙连接通道的物理性质决定了线粒体无法依此途径出胞,故是线粒体膜上的缝隙连接蛋白43六聚体参与运输机制调控,还是有更丰富的缝隙连接蛋白43活化形式共同诱导其迁移,需要继续探究。 缝隙连接蛋白43的细胞外囊泡定位:细胞外囊泡是生物信息的主要载体,在生理和病理条件下介导局部和全身细胞间的通信。这些内源性囊泡已被公认为各种治疗药物的主要递送载体。一般认为,其可以携带多种活性大分子,包括蛋白质、代谢物、脂质和核酸等 [69],从而参与多种肿瘤、神经系统疾病和心血管疾病的病理过程[70]。而缝隙连接蛋白43被发现以六聚体形式定位于细胞外囊泡膜表面,形成半通道样结构,从而游走迁移于细胞外基质中,完成远距离信号递送 [71]。其促进细胞外囊泡与受体细胞的对接,以一种高效实时动态的新型连接方式进行细胞间通讯[72],为细胞外囊泡的摄取机制研究提供了有利的理论依据。在抗肿瘤靶向药物研究中也展现了其优越性,与脂质体或游离药物相比,使用含有功能性缝隙连接蛋白43通道的细胞外囊泡递送化疗药[73],可以显著提高其治疗效果。MARTINS-MARQUES团队[74]通过细胞外囊泡包裹阿霉素后行抗肿瘤治疗,发现其还可以降低药物的心脏毒性。总的来说,缝隙连接蛋白43参与细胞外囊泡靶向迁移的调控机制虽尚不明确,但现有研究已显示了其客观疗效的众多可能性。 2.3.3 选择性截短缝隙连接蛋白43片段的独特作用 除了全长的缝隙连接蛋白43之外,仍存在由内源性选择性翻译产生的缝隙连接蛋白43片段,其自身可以游走于胞内,与不同形式缝隙连接蛋白43相互作用,构成完整的缝隙连接蛋白43互作机制网络。该片段型缝隙连接蛋白43的出现与其独特的遗传密码密切相关,即缝隙连接蛋白43 mRNA是以多顺反子的形式存在,在上游存在总共7个蛋氨酸起始密码子,因此可翻译获得7种类型缝隙连接蛋白43;除全长缝隙连接蛋白43外,其他6种相对分子质量分别为32 000,29 000,26 000,20 000,11 000和7 000,前3种包含2个跨膜结构域、部分细胞内环和C末端,20-kD异构体(GJA1-20k) 包含第4个跨膜结构域的一部分和C末端,后两种仅具有完整C末端结构。与常规连接蛋白的命名不同,该亚型采用缝隙连接蛋白43基因名称(GJA1)与截短蛋白大小相结合的方法,从而直观地反映了mRNA的来源。虽然氨基端都有不同程度的截短,但包含主要功能形式的羧基末端都具有较好完整性,因此仍保持丰富的生物学活性[75]。 全长缝隙连接蛋白43与选择性翻译片段间存在高度串扰的交通网络。研究显示了GJA1-20k亚型在人类心脏组织、斑马鱼心脏组织和许多癌细胞系中的表达占主导地位[76-79],其可与全长缝隙连接蛋白43相互作用,通过与微管结合,调控缝隙连接蛋白43向质膜运输,从而促进缝隙连接的形成和细胞间通讯[76]。在引入突变的GJA1-20k亚型前后[75],缝隙连接形成也呈正向相关性改变,这初步表明GJA1-20k亚型可能作为蛋白伴侣协助缝隙连接蛋白43以囊泡形式的靶向运输过程。但至今关于片段型缝隙连接蛋白43参与肌动蛋白主导的全长缝隙连接蛋白43靶向运输的具体机制尚不完全清楚,其如何调节微管与肌动蛋白,从而精准靶向运输缝隙连接蛋白43到各处亚结构域,则需要更加完善的实验进一步探究。 同时,GJA1-20k亦以线粒体为靶点,调节线粒体的靶向运输及功能代谢。线粒体主要负责能量生产、钙稳态、细胞信号传导、细胞生长和存活等。GJA1-20k突变株(GJA1-20k-del6)缺乏结合微管的能力[76],但却仍能到达线粒体膜,说明了与全长缝隙连接蛋白43转运机制不同,在缝隙连接蛋白43通过肌动蛋白沿微管进行靶向运输以前,GJA1-20k有可能已经定位于线粒体膜,这或许为线粒体编码缝隙连接蛋白43提供了研究的可能性,并提示多来源定位的GJA1-20k可能承担不同作用。在调节线粒体功能代谢方面,发现细胞在氧化应激条件下,GJA1-20k可以促进线粒体周围肌动蛋白的聚合,形成焦点收缩位点以启动线粒体裂变[80-81]。此现象为细胞自我保护机制,还是加重了线粒体-细胞核相互诱导的损伤,在多类代谢性疾病机理研究中至关重要。"
| [1] SU V, LAU AF. Connexins: mechanisms regulating protein levels and intercellular communication. FEBS Lett. 2014;588(8):1212-1220. [2] YEAGER M, HARRIS AL. Gap junction channel structure in the early 21st century: facts and fantasies. Curr Opin Cell Biol. 2007;19(5):521-528. [3] KELSELL DP, DUNLOP J, HODGINS MB. Human diseases: clues to cracking the connexin code. Trends Cell Biol. 2001;11(1):2-6. [4] ZHENG H, LIU Y, XU D, et al. Inhibition of gap junction-mediated intercellular communication by Poly (I:C) in cultured human corneal fibroblasts. Curr Eye Res. 2020;45(9):1043-1050. [5] JIANG J, HOAGLAND D, PALATINUS JA, et al. Interaction of α Carboxyl terminus 1 peptide with the connexin 43 carboxyl terminus preserves left ventricular function after ischemia-reperfusion injury. J Am Heart Assoc. 2019;8(16):e012385. [6] BEYER EC, BERTHOUD VM. Gap junction gene and protein families: connexins, innexins, and pannexins. Biochim Biophys Acta Biomembr. 2018;1860(1):5-8. [7] OYAMADA M, TAKEBE K, ENDO A, et al. Connexin expression and gap-junctional intercellular communication in ES cells and iPS cells. Front Pharmacol. 2013;4:85. [8] NOORMAN M, VAN DER HEYDEN MA, VAN VEEN TA, et al. Cardiac cell-cell junctions in health and disease: electrical versus mechanical coupling. J Mol Cell Cardiol. 2009;47(1):23-31. [9] SMYTH JW, SHAW RM. The gap junction life cycle. Heart Rhythm. 2012;9(1):151-153. [10] SOLAN JL, LAMPE PD. Specific Cx43 phosphorylation events regulate gap junction turnover in vivo. FEBS Lett. 2014;588(8):1423-1429. [11] OSHIMA A, TANI K, HIROAKI Y, et al. Three-dimensional structure of a human connexin26 gap junction channel reveals a plug in the vestibule. Proc Natl Acad Sci U S A. 2007;104(24):10034-10039. [12] EPIFANTSEVA I, SHAW RM. Intracellular trafficking pathways of Cx43 gap junction channels. Biochim Biophys Acta Biomembr. 2018;1860(1):40-47. [13] MARTINS-MARQUES TANIA, RIBEIRO-RODRIGUES TERESA, BATISTA-ALMEIDA DANIELA, et al. Biological functions of connexin43 beyond intercellular communication. Trends Cell Biol. 2019;29(10):835-847. [14] BEYER EC, PAUL DL, GOODENOUGH DA. Connexin 43: a protein from rat heart homologous to a gap junction protein from liver. J Cell Biol. 1987;105(6 Pt 1): 2621-2629. [15] RUSIECKA OM, MONTGOMERY J, MOREL S, et al. Canonical and non-canonical roles of connexin43 in cardioprotection. biomolecules. 2020;10(9):1225. [16] MÁRQUEZ-ROSADO L, SOLAN JL, DUNN CA, et al. Connexin43 phosphorylation in brain, cardiac, endothelial and epithelial tissues. Biochim Biophys Acta. 2012; 1818(8):1985-1992. [17] BOENGLER K, ROHRBACH S, WEISSMANN N, et al. Importance of Cx43 for right ventricular function. Int J Mol Sci. 2021;22(3):987. [18] UNE H, YAMASAKI R, NAGATA S, et al. Brain gray matter astroglia-specific connexin 43 ablation attenuates spinal cord inflammatory demyelination. J Neuroinflammation. 2021;18(1):126. [19] GRUBER HE, MA D, HANLEY EN JR, et al. Morphologic and molecular evidence for gap junctions and connexin 43 and 45 expression in annulus fibrosus cells from the human intervertebral disc. J Orthop Res. 2001;19(5):985-989. [20] BERKOVITZ BK, BECKER D. Detailed morphology and distribution of gap junction protein associated with cells from the intra-articular disc of the rat temporomandibular joint. Connect Tissue Res. 2003;44(1):12-18. [21] 张婧.实验性咬合紊乱致大鼠TMJ髁突骨关节炎样变及Cx43半通道在关节软骨退变中的作用[D].西安:第四军医大学,2013. [22] YANG Y, LIU W, WEI J, et al. Transforming growth factor-β1-induced N-cadherin drives cell-cell communication through connexin43 in osteoblast lineage. Int J Oral Sci. 2021;13(1):15. [23] TALBOT J, BRION R, LAMORA A, et al. Connexin43 intercellular communication drives the early differentiation of human bone marrow stromal cells into osteoblasts. J Cell Physiol. 2018;233(2):946-957. [24] TALBOT J, DUPUY M, MORICE S, et al. Antagonistic functions of connexin 43 during the development of primary or secondary bone tumors. Biomolecules. 2020;10(9):1240. [25] 沈斌,韦卉,任茜,等.缝隙连接蛋白43与肿瘤关系的研究进展[J].医学综述,2017,23(20):4001-4006. [26] KAR R, BATRA N, RIQUELME MA, et al.Biological role of connexin intercellular channels and hemichannels. Arch Biochem Biophys. 2012;524(1):2-15. [27] LAIRD DW. Syndromic and non-syndromic disease-linked Cx43 mutations. FEBS Lett. 2014;588(8):1339-1348. [28] MEŞE G, RICHARD G, WHITE TW. Gap junctions: basic structure and function. J Invest Dermatol. 2007;127(11):2516-2524. [29] GOODENOUGH DA, PAUL DL. Gap junctions. Cold Spring Harb Perspect Biol. 2009;1(1):a002576. [30] KANAPORIS G, MESE G, VALIUNIENE L, et al. Gap junction channels exhibit connexin-specific permeability to cyclic nucleotides. J Gen Physiol. 2008;131(4): 293-305. [31] MCCAIN ML, DESPLANTEZ T, GEISSE NA, et al. Cell-to-cell coupling in engineered pairs of rat ventricular cardiomyocytes: relation between Cx43 immunofluorescence and intercellular electrical conductance. Am J Physiol Heart Circ Physiol. 2012;302(2):H443-H450. [32] ALONSO F, KRATTINGER N, MAZZOLAI L, et al. An angiotensin II- and NF-kappaB-dependent mechanism increases connexin 43 in murine arteries targeted by renin-dependent hypertension. Cardiovasc Res. 2010;87(1):166-176. [33] 唐良虎,李德,杨大春,等.上调Cx43对心肌细胞间通讯及离子通道的影响[J].西部医学,2016,28(3):6. [34] COTRINA ML, LIN JH, NEDERGAARD M. Adhesive properties of connexin hemichannels. Glia. 2008;56(16):1791-1798. [35] LIN JH, TAKANO T, COTRINA ML, et al. Connexin 43 enhances the adhesivity and mediates the invasion of malignant glioma cells. J Neurosci. 2002;22(11):4302-4311. [36] ELIAS LA, WANG DD, KRIEGSTEIN AR. Gap junction adhesion is necessary for radial migration in the neocortex. Nature. 2007;448(7156):901-907. [37] 赵希伟,周佳伟,刘凯,等.连接蛋白43通过蛋白激酶A介导丝氨酸373调控脓毒症急性肺损伤肺泡Ⅱ型上皮细胞屏障功能的研究[J].中华危重症医学杂志(电子版),2021,14(5):355-361. [38] NAGASAWA K, CHIBA H, FUJITA H, et al. Possible involvement of gap junctions in the barrier function of tight junctions of brain and lung endothelial cells. J Cell Physiol. 2006;208(1):123-132. [39] HARRIS AL, BEVANS CG. Exploring hemichannel permeability in vitro. Methods Mol Biol. 2001;154:357-377. [40] GUTSTEIN DE, MORLEY GE, TAMADDON H, et al.Conduction slowing and sudden arrhythmic death in mice with cardiac-restricted inactivation of connexin 43. Circ Res. 2001;88(3):333-339. [41] XING L, YANG T, CUI S, et al. Connexin hemichannels in astrocytes: role in CNS disorders. Front Mol Neurosci. 2019;12:23. [42] KAMERITSCH P, POGODA K. The role of connexin 43 and pannexin 1 during acute inflammation. Front Physiol. 2020;11:594097. [43] CALDER BW, MATTHEW RHETT J, BAINBRIDGE H, et al. Inhibition of connexin 43 hemichannel-mediated ATP release attenuates early inflammation during the foreign body response. Tissue Eng Part A. 2015;21(11-12):1752-1762. [44] DOSCH M, ZINDEL J, JEBBAWI F, et al. Connexin-43-dependent ATP release mediates macrophage activation during sepsis. Elife. 2019;8:e42670. [45] WILLEBRORDS J, CRESPO YANGUAS S, MAES M, et al. Connexins and their channels in inflammation. Crit Rev Biochem Mol Biol. 2016;51(6):413-439. [46] KELLEY N, JELTEMA D, DUAN Y, et al. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. 2019;20(13):3328. [47] ZHOU KQ, GREEN CR, BENNET L, et al. The role of connexin and pannexin channels in perinatal brain injury and inflammation. Front Physiol. 2019;10:141. [48] YANG Y, WANG H, KOUADIR M,et al. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019;10(2):128. [49] PRICE GW, CHADJICHRISTOS CE, KAVVADAS P, et al. Blocking Connexin-43 mediated hemichannel activity protects against early tubular injury in experimental chronic kidney disease. Cell Commun Signal. 2020;18(1):79. [50] MUGISHO OO, GREEN CR, ZHANG J, et al. Connexin43 hemichannels: a potential drug target for the treatment of diabetic retinopathy. Drug Discov Today. 2019; 24(8):1627-1636. [51] 薛俊杰,李婧瑜,张莉,等.缝隙连接蛋白43在骨关节炎软骨及细胞中表达及shRNA慢病毒载体的构建[J].中国组织工程研究,2020,24(23):3627-3635. [52] 蔡正旭.由CX32和CX43组成的缝隙连接在癫痫发病中的作用及调控机制的实验研究[D].长春:吉林大学,2004. [53] RODJAKOVIC D, SALM L, BELDI G. Function of connexin-43 in macrophages. Int J Mol Sci. 2021;22(3):1412. [54] WILLEBRORDS J, MAES M, CRESPO YANGUAS S, et al.Inhibitors of connexin and pannexin channels as potential therapeutics. Pharmacol Ther. 2017;180:144-160. [55] BOENGLER K, DODONI G, RODRIGUEZ-SINOVAS A, et al. Connexin 43 in cardiomyocyte mitochondria and its increase by ischemic preconditioning. Cardiovasc Res. 2005;67(2):234-244. [56] KOZORIZ MG, CHURCH J, OZOG MA, et al.Temporary sequestration of potassium by mitochondria in astrocytes. J Biol Chem. 2010;285(41):31107-31119. [57] LI H, BRODSKY S, KUMARI S, et al. Paradoxical overexpression and translocation of connexin43 in homocysteine-treated endothelial cells. Am J Physiol Heart Circ Physiol. 2002;282(6):H2124-H2133. [58] KIM SN, KWON HJ, IM SW, et al. Connexin 43 is required for the maintenance of mitochondrial integrity in brown adipose tissue. Sci Rep. 2017;7(1):7159. [59] BOENGLER K, SCHULZ R. Connexin 43 and mitochondria in cardiovascular health and disease. Adv Exp Med Biol. 2017;982:227-246. [60] GADICHERLA AK, WANG N, BULIC M, et al. Mitochondrial Cx43 hemichannels contribute to mitochondrial calcium entry and cell death in the heart. Basic Res Cardiol. 2017;112(3):27. [61] WANG M, SMITH K, YU Q, et al. Mitochondrial connexin 43 in sex-dependent myocardial responses and estrogen-mediated cardiac protection following acute ischemia/reperfusion injury. Basic Res Cardiol. 2019;115(1):1. [62] GUO R, SI R, SCOTT BT, et al. Mitochondrial connexin40 regulates mitochondrial calcium uptake in coronary endothelial cells. Am J Physiol Cell Physiol. 2017; 312(4):C398-C406. [63] BOENGLER K, HEUSCH G, SCHULZ R. Connexin 43 and ischemic preconditioning: effects of age and disease. Exp Gerontol. 2006;41(5):485-488. [64] FERNANDEZ-SANZ C, RUIZ-MEANA M, CASTELLANO J, et al. Altered FoF1 ATP synthase and susceptibility to mitochondrial permeability transition pore during ischaemia and reperfusion in aging cardiomyocytes. Thromb Haemost. 2015;113(3):441-451. [65] LIU D, GAO Y, LIU J, et al. Intercellular mitochondrial transfer as a means of tissue revitalization. Signal Transduct Target Ther. 2021;6(1):65. [66] ISLAM MN, DAS SR, EMIN MT, et al. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18(5):759-765. [67] YAO Y, FAN XL, JIANG D, et al. Connexin 43-mediated mitochondrial transfer of iPSC-MSCs alleviates asthma inflammation. Stem Cell Reports. 2018;11(5):1120-1135. [68] GOLAN K, SINGH AK, KOLLET O, et al. Bone marrow regeneration requires mitochondrial transfer from donor Cx43-expressing hematopoietic progenitors to stroma. Blood. 2020;136(23):2607-2619. [69] SANTANGELO L, GIURATO G, CICCHINI C, et al. The RNA-binding protein SYNCRIP is a component of the hepatocyte exosomal machinery controlling microrna sorting. Cell Rep. 2016;17(3):799-808. [70] LIN YN, MESQUITA T, SANCHEZ L, et al. Extracellular vesicles from immortalized cardiosphere-derived cells attenuate arrhythmogenic cardiomyopathy in desmoglein-2 mutant mice. Eur Heart J. 2021;42(35):3558-3571. [71] COLOMBO M, RAPOSO G, THÉRY C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255-289. [72] SOARES AR, MARTINS-MARQUES T, RIBEIRO-RODRIGUES T, et al. Gap junctional protein Cx43 is involved in the communication between extracellular vesicles and mammalian cells. Sci Rep. 2015;5:13243. [73] GADOK AK, BUSCH DJ, FERRATI S, et al. Connectosomes for direct molecular delivery to the cellular cytoplasm. J Am Chem Soc. 2016;138(39):12833-12840. [74] MARTINS-MARQUES T, PINHO MJ, ZUZARTE M, et al. Presence of Cx43 in extracellular vesicles reduces the cardiotoxicity of the anti-tumour therapeutic approach with doxorubicin. J Extracell Vesicles. 2016;5:32538. [75] SMYTH JW, SHAW RM. Autoregulation of connexin43 gap junction formation by internally translated isoforms. Cell Rep. 2013;5(3):611-618. [76] BASHEER WA, XIAO S, EPIFANTSEVA I, et al. GJA1-20k Arranges Actin to Guide Cx43 Delivery to Cardiac Intercalated Discs. Circ Res. 2017; 121(9):1069-1080. [77] JAMES CC, ZEITZ MJ, CALHOUN PJ, et al. Altered translation initiation of Gja1 limits gap junction formation during epithelial-mesenchymal transition. Mol Biol Cell. 2018;29(7):797-808. [78] SALAT-CANELA C, SESÉ M, PEULA C, et al. Internal translation of the connexin 43 transcript. Cell Commun Signal. 2014;12:31. [79] CHATTERJEE B, CHIN AJ, VALDIMARSSON G, et al. Developmental regulation and expression of the zebrafish connexin 43 gene. Dev Dyn. 2005;233(3):890-906. [80] FU Y, ZHANG SS, XIAO S, et al. Cx43 Isoform GJA1-20k Promotes Microtubule Dependent Mitochondrial Transport. Front Physiol. 2017;8:905. [81] SHIMURA D, NUEBEL E, BAUM R, et al. Protective mitochondrial fission induced by stress-responsive protein GJA1-20k. Elife. 2021;10:e69207. |
| [1] | Feng Ruiqin, Han Na, Zhang Meng, Gu Xinyi, Zhang Fengshi. Combination of 1% platelet-rich plasma and bone marrow mesenchymal stem cells improves the recovery of peripheral nerve injury [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 985-992. |
| [2] | Liu Qiwei, Zhang Junhui, Yang Yuan, Wang Jinjuan. Role and mechanism of umbilical cord mesenchymal stem cells on polycystic ovary syndrome [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1015-1020. |
| [3] | Sun Teng, Han Yu, Wang Shuang, Li Jialei, Cao Jimin. miR-20a regulates pressure overload-induced cardiac hypertrophy [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1021-1028. |
| [4] | Liu Jianhong, Liao Shijie, Li Boxiang, Tang Shengping, Wei Zhendi, Ding Xiaofei. Extracellular vesicles carrying non-coding RNA regulate the activation of osteoclasts [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1076-1082. |
| [5] | Pan Xiaolong, Fan Feiyan, Ying Chunmiao, Liu Feixiang, Zhang Yunke. Effect and mechanism of traditional Chinese medicine on inhibiting the aging of mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1091-1098. |
| [6] | Liu Hanfeng, Wang Jingjing, Yu Yunsheng. Artificial exosomes in treatment of myocardial infarction: current status and prospects [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1118-1123. |
| [7] | Long Yi, Yang Jiaming, Ye Hua, Zhong Yanbiao, Wang Maoyuan. Extracellular vesicles in sarcopenic obesity: roles and mechanisms [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(2): 315-320. |
| [8] | Zhang Yongqiang, Pan Feng, Sun Peng, Tan Minghui, Xuan Liuming, Wang Qinzhang. Effect of Gja1 gene recombinant lentivirus on Cx43 protein and mRNA expression in a diabetic guinea pig bladder model [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1161-1165. |
| [9] | Jia Shengqi, Luo Wenlong, Tian Dingyuan, Zhang Xinhui, Cui Qian, Wang Chao, Pei Hanjun. Expression of mitochondrial sirtuin 3 in mice with acute renal ischemia-reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1172-1178. |
| [10] | Ding Yiqun, Li Xigong, Pan Wenming, Zhang Qin. Role and advance of mitophagy in spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(35): 5727-5733. |
| [11] | He Qi, Pan Zhaofeng, Chen Baihao, Yang Junzheng, Li Shaocong, Zeng Jiaxu, Zhou Chi, Wang Haibin. Establishment of chondrocyte model of iron overload and the mechanism of injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(32): 5126-5132. |
| [12] | Dong Jiaan, Liu Ruyin, Yue Zongjin, Xu Xiangyang, Wang Zhengzhen. Huangqi Guizhi Wuwu Decoction attenuates mitochondrial damage and oxidative stress in intervertebral disc endplate chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(32): 5137-5143. |
| [13] | Jiang Shengyuan, Zhang Yaqi, Bi Jingxin, Zhang Chengfei, Zhang Qiue, Mu Xiaohong, Bai Huizhong, Zuo Xinwei, Liu Tonghua, Qin Lingling. Tangbikang reduces sciatic nerve oxidative stress injury in a rat model of diabetic peripheral neuropathy [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(32): 5162-5167. |
| [14] | Peng Fengli, Li Chaofu, Shi Bei. Application of the nanovesicle delivery system in cardiovascular diseases [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(30): 4862-4868. |
| [15] | Cao Zhipeng, Shi Yu, Li Ke, Lin Wenzheng, Jiang Letao, Bu Wenzhen, Zhu Hai, Du Jianwei, Wang Huihui, Chen Hao. Carbon dot Prussian blue nanoenzyme antioxidative stress delays intervertebral disc degeneration [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(25): 4038-4044. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||