Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (7): 1118-1123.doi: 10.12307/2024.111
Previous Articles Next Articles
Artificial exosomes in treatment of myocardial infarction: current status and prospects
Liu Hanfeng, Wang Jingjing, Yu Yunsheng
- Department of Cardiovascular Surgery, First Affiliated Hospital, Soochow University, Suzhou 215000, Jiangsu Province, China
-
Received:2023-01-12Accepted:2023-03-23Online:2024-03-08Published:2023-07-17 -
Contact:Yu Yunsheng, MD, Chief physician, Associate professor, Master’s supervisor, Department of Cardiovascular Surgery, First Affiliated Hospital, Soochow University, Suzhou 215000, Jiangsu Province, China -
About author:Liu Hanfeng, Master candidate, Department of Cardiovascular Surgery, First Affiliated Hospital, Soochow University, Suzhou 215000, Jiangsu Province, China -
Supported by:Scientific Research Project of Jiangsu Provincial Health Commission, No. ZDA2020017 (to YYS)
CLC Number:
Cite this article
Liu Hanfeng, Wang Jingjing, Yu Yunsheng. Artificial exosomes in treatment of myocardial infarction: current status and prospects[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1118-1123.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
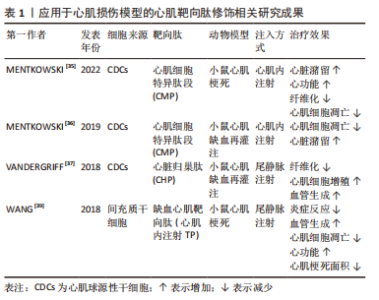
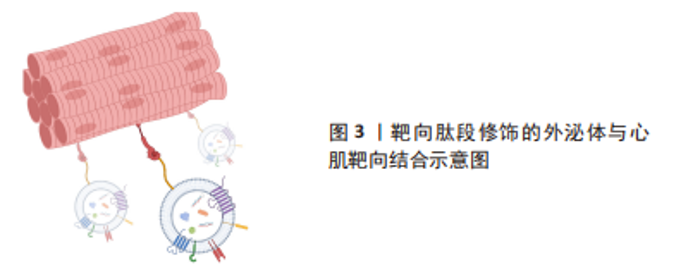
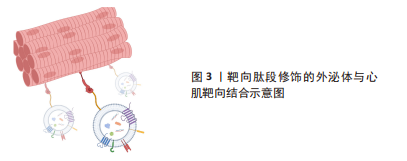
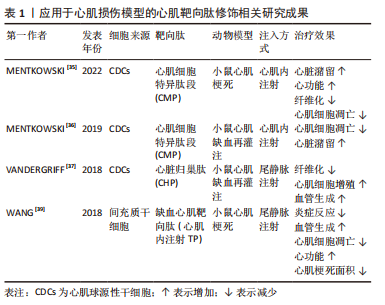
尽管在过去的几十年里,外泌体在各种治疗和诊断方面的应用越来越受欢迎,但要想在生物医学领域充分挖掘其潜力,仍有许多问题需要阐明。天然外泌体的使用受到限制是因为其药物装载能力有限,临床使用的产量不足,生物分布有限,靶向性取决于母体细胞,此外还有一些脱靶效应[24-26]。鉴于天然外泌体的这些使用局限性,人们意识到需要对外泌体进行工程设计,以进一步提高内容物质量,改善治疗方法[27-33]。工程化的方法有助于提高外泌体的可扩展性,并扩大外泌体在其固有能力之外的生物医学应用范围[34]。对于如何将外泌体进行工程化以提高在心肌梗死中的治疗效果,目前有两种不同的思路。一种是在天然来源的外泌体的基础上对其加以改造包括:①对外泌体的表面进行各种大分子修饰,如多肽、染料等进行细胞跟踪或者靶向特定的细胞或组织;②对亲代细胞进行改造以产生具有靶向肽段或治疗性内容物的人造外泌体;③通过挤压、共孵育、冻融、电转和超声等方式对外泌体进行杂交和治疗性药物的装载,对此思路,研究者们将其称为半人造外泌体,其已经逐渐成为目前的主流外泌体改造模式,但是由于这一想法仍然是基于天然来源的外泌体的改造,因此还是无法避免要获取大量天然外泌体并对其进行繁杂的表征鉴定以及面对天然来源外泌体苛刻的存储条件。而另一种思路则是以天然外泌体为模板,构建一种仿生纳米颗粒也就是全人造外泌体,这样的纳米颗粒可以作为一种高效的药物递送系统用于心肌梗死的治疗中,并且具有相当的生物相容性、可编辑性及大规模生产的潜力。 2.1 半人造外泌体 2.1.1 靶向肽修饰 为了增强天然外泌体在心血管疾病中的治疗效果,调节改善其生物活性、稳定性、靶向性,研究人员分别在外泌体分泌前后对外泌体分泌细胞或外泌体进行不同处理,其中对外泌体表面进行靶向肽段(归巢肽)的修饰是目前的研究热点之一。通过锚定归巢肽修饰外泌体膜是解决非特异性递送的一种方法。组织特异性归巢肽通常通过体内筛选噬菌体展示形成的肽库来鉴定。归巢肽的设计特别取决于血管的异质性和病理生理条件。当归巢肽应用于外泌体时,由于外泌体表面组成的改变,它们可以改变外泌体的特异性。因此,研究人员已经研究了各种不通过改变特定的天然外泌体蛋白质或脂质来改变外泌体表面的归巢肽结合方法。例如,通过基因克隆技术制备归巢肽与外泌体蛋白的融合蛋白,由于其高产量和广泛性而得到了广泛的应用。 为了制备融合蛋白,将归巢肽序列插入编码外泌体表面蛋白的基因中。另外,将归巢肽锚定到外泌体表面是另一种方法。例如,已知中性磷脂、二肉豆蔻酰磷脂酰乙醇胺缀合的归巢肽能够迅速与外泌体脂质双层融合[40-41]。该文章作者及其研究团队前期的研究显示缺血心肌靶向肽IMTP(CSTSMLKAC)的融合可以赋予外泌体对缺血心肌的显著特异性和效率。同时,间充质干细胞衍生的IMTP外泌体在小鼠心肌梗死模型中对抑制炎症、增强血管生成和心脏功能具有显著作用。因此,缺血心肌特异性肽序列工程外泌体代表了一种新的分子工具,可用于靶向和治疗缺血心肌[38-39]。另一项研究使用心肌细胞靶向肽(APWHLSSQYSRT)生产制造外泌体表面蛋白融合归巢肽[42]。研究人员使用HEK293细胞制造了人造外泌体,表达归巢肽的外泌体在体外优先递送到大鼠心肌细胞和体内小鼠梗死心肌,并且没有明显的毒性。此外,他们通过添加糖基化基序来修饰靶向肽序列的N端,该基序保护靶向肽不被降解,从而增强体内半衰期和心肌特异性外泌体递送。表1列举了近年来应用于心肌损伤模型的心肌靶向肽。通过修饰外泌体膜,这些蛋白质可以与梗死心肌中表达的特定细胞受体或细胞外基质成分相互作用,见图3。虽然许多研究没有量化绝对外泌体积累,但结果表明新进靶向肽修饰可以增加外泌体对心肌缺血区域的趋向性,并增强外泌体的治疗效果[37,39,43]。"
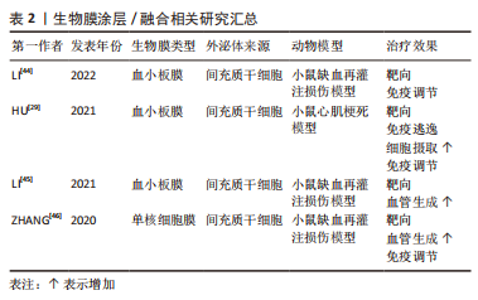
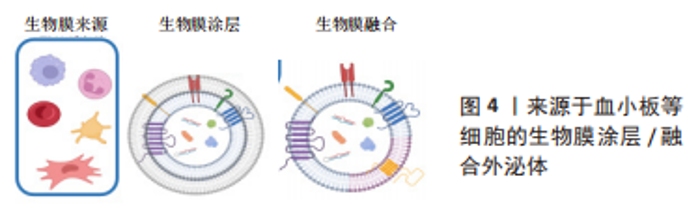
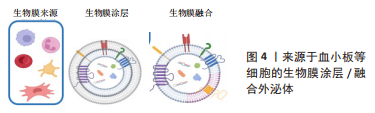
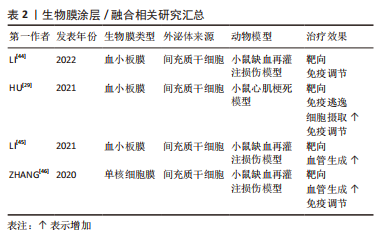
2.1.2 生物膜涂层/膜融合 细胞膜最近已成为药物递送系统的新型仿生材料来源。这些细胞膜常被挤出或超声处理以制造成纳米级囊泡[47]。与合成脂质或聚合物纳米颗粒不同,细胞膜衍生的囊泡具有独特的多组分特征,包括脂质、蛋白质和碳水化合物。由于细胞膜衍生的囊泡包含其亲代细胞的内在功能和信号网络,它们可以克服体内遇到的各种障碍,例如,细胞膜表面可能含有“Don’t eat me”受体CD47和CD24[48-49],它们可以通过减少网状内皮系统细胞的摄取来延长人造外泌体的循环时间。此外,来自不同细胞来源的不同天然膜组合扩大了细胞膜衍生囊泡的范围,创造了一种全新的药物递送系统类别[50]。细胞膜衍生的囊泡可以在其内部携带治疗剂,也可以覆盖载药核心纳米颗粒的表面。细胞膜通常来自单细胞来源,包括红细胞[51-52]、血小板[45-53]、免疫细 胞[46,54]、干细胞和癌细胞[55-56]。利用不同细胞来源的细胞膜所具有的特性及良好的生物相容性,将其与外泌体相结合有望成为一种新型纳米药物递送系统。 如表2和图4所示,血小板膜是嵌有糖蛋白整合素的脂质双分子层。它们具有膜结合分子,如 GPIIb/IIIa,CD62P,免疫调节蛋白,CD47和 CD55;它们还具有跨膜蛋白 GPIbα, GPIV, GPVI 和 GPIX[57]。与血小板膜结合后,间充质干细胞来源的外泌体被内皮细胞、心肌细胞的摄取显著增加,而其被单核巨噬细胞系统的摄取下降。同时人造的外泌体显示出与受损血管内皮更强的结合力,提高了对缺血心肌的靶向能力[53]。LI等[44]使用血小板膜修饰间充质干细胞来源的外泌体,使其靶向结合缺血心脏损伤的内皮细胞,并且增强了外泌体在心肌梗死区域的促血管生成能力。自2015年张连方教授及其同事首次将血小板膜作为纳米生物涂层以来[53],生物膜纳米涂层技术已取得了许多进展,但该领域的研究仍处于起步阶段,在这些纳米粒子从实验室到临床阶段过渡之前,还需要克服几个挑战。与生物膜涂层纳米颗粒相关的主要挑战是:制造过程的复杂性和可重复性问题;不同类型生物膜涂层纳米颗粒疗效的异质性;这些物理和化学融合的细胞膜的稳定性也存在问题。然而,到目前为止所进行的工作表明,生物膜涂层纳米颗粒在提供新兴的新型治疗和诊断模式以及革新纳米医学来治疗无数重要的人类疾病方面具有巨大的前景。"
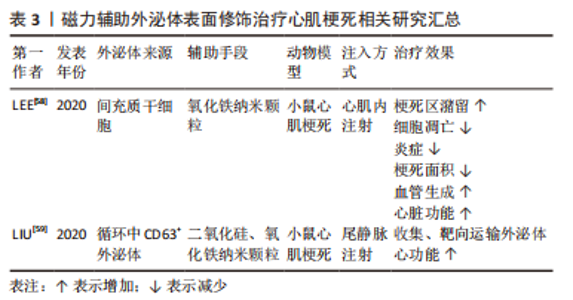
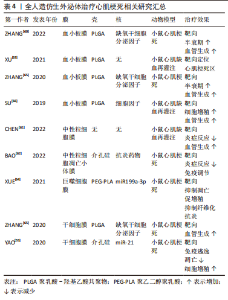
2.1.3 磁力辅助 无论是在外泌体表面修饰心脏靶向肽或者融合/涂层生物膜,目前的活体实验大多通过小鼠尾静脉注射的方式进行治疗,而研究发现这种方式使得人造的外泌体易于在肝脏、脾脏、肺脏中非选择性积累,或在外周血中被单核巨噬细胞系统清除,这将会造成治疗效果不佳,甚至对无关器官造成毒性及外泌体的浪费。将磁力物质如纳米氧化铁与外泌体相结合,通过在体外以磁力制导的方式使改造的外泌体靶向目标位置,可以实现产品的高靶向率和潴留率[59-60],见表3。LEE等[58]将氧化铁纳米颗粒(iron oxide magnetic nanoparticles,IONPs)内化到间充质干细胞中,通过滤过膜细胞连续挤出技术制备了外泌体大小的含IONP纳米囊泡(IONP Nano Vesicles,IONP NVs),其内包含的活性蛋白及RNA等成分与间充质干细胞分泌的天然外泌体相似且较后者含量更多,同样的具有外泌体特征的表面标志物;在体外他们发现,与间充质干细胞相似,IONP NVs对心脏成纤维细胞、心肌细胞、内皮细胞和巨噬细胞表现出抗凋亡、抗纤维化、抗炎和促血管生成作用。"
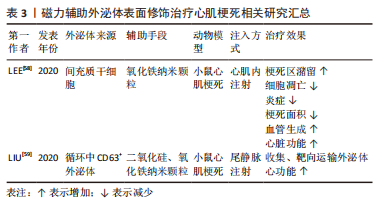
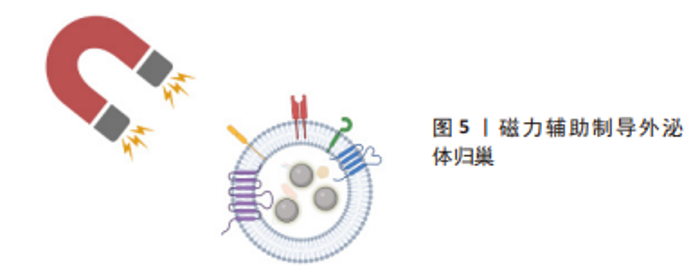
在大鼠心肌梗死模型中,通过在心肌梗死区心肌内注射IONP NVs,磁引导24 h后,IONP NVs在损伤部位的保留显著增强并通过抗凋亡、抗炎、血管生成和抗纤维化促进心脏修复。不同于Lee的团队将纳米氧化铁内化到外泌体内部的策略,LIU等[59]设计并制造了一种由氧化铁核和二氧化硅壳组成的纳米颗粒,并在其表面结合了能与外泌体特异性结合的抗CD63抗体以及与受损心肌细胞特异性结合的抗MLC抗体。这一磁性纳米颗粒在静脉注射后捕获外周血中CD63阳性外泌体并在磁场的引导下运往心肌梗死区,然后在酸性环境中释放外泌体从而达到心肌梗死模型中梗死面积的减小以及左心室射血分数和血管生成的改善。利用磁场的磁力制导作用可以方便、高效地将活性成分靶向递送至目的区域,避免了对外泌体进行繁琐复杂的改性,有效地延长了外泌体的循环和潴留时间,见图5。未来在对外泌体的表征及在人体内的作用机制有了更透彻的理解后,磁力辅助将会是一种重要的设计方向,但同时磁力物质会不会在体内造成不必要的扩散从而导致一些不良的后果需要后续的深入研究来解答。"
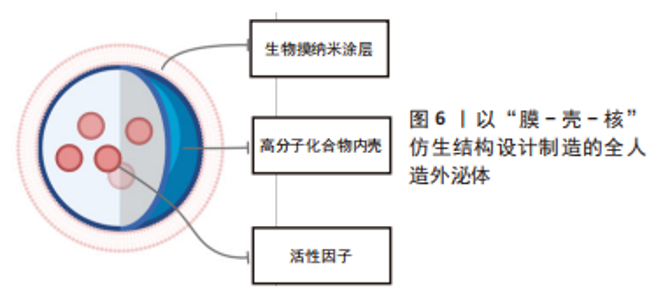
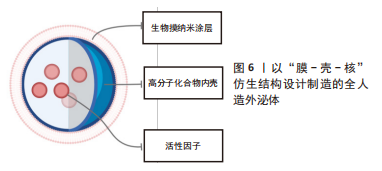
2.2 全人造外泌体 外泌体携带多种复杂的配体和受体,包括疾病相关蛋白,并在物理和病理过程中发挥多方面的作用。然而,其潜在机制远未完全了解,这可能会带来潜在的安全问题,从而阻碍外泌体向临床的转化。作为获得天然外泌体优势特征同时规避相当复杂性和潜在安全问题的初步解决方案,研究人员综合设计了模拟外泌体的材料。从概念上讲,完全人造的类外泌体纳米颗粒是指受天然外泌体启发而装饰有功能性膜蛋白的合成脂质双分子层,这些纳米囊泡是在严格控制的实验室条件下由特征良好的生物分子(即蛋白质和脂质)制备的,因此具有更高的药学可接受性[67]。合成构建模拟外泌体的仿生材料的前提是,并非天然外泌体中的所有成分对于外泌体的正常功能和递送特性都是必不可少的。根据特定的治疗目的,仅将来自天然外泌体的关键成分掺入人工纳米颗粒中可以赋予它们强大的生物学功能和有效的细胞内递送效率,但不像它们的生物学对应物那么复杂。 人造外泌体样纳米颗粒通常通过两个步骤构建:模仿天然外泌体组装脂质双层,以及用功能性膜蛋白对脂质表面进行功能化。特别是,目前大多数人工外泌体样纳米颗粒是脂质体和脂质双层伪装的纳米颗粒,其膜组成与天然外泌体相似。从结构的角度来看,脂质体与天然外泌体共享球形脂质双层结构,以及可调节的直径和脂质组成,因此可作为仿生外泌体类纳米颗粒合成组装的理想原始结构。除了特定的脂质外,外泌体的许多有益特性源于其表面生物活性蛋白的表达。 文章列举了以生物膜作为纳米颗粒表面涂层的人造外泌体在心肌梗死治疗中的应用。这类产品的设计思路大多以膜-壳-核的类细胞结构为基础,见表4和图6,再根据具体需要进行相应地改造修饰。如前所述,选择生物膜作为人造外泌体的表面涂层使其具有良好的生物相容性并且生物膜保留有母细胞所具有的功能性结构,使人造外泌体得到相应的功能继承;而生物膜所具有的天然磷脂双分子层可以相对容易地通过如化学点击、共价结合等方式进行表面改性,使其具有更为丰富的特性。细胞骨架在细胞和有机体水平的许多基本过程中发挥着重要作用,包括细胞迁移和运动、细胞分裂以及细胞和组织结构的建立和维持[68]。通过使用具有一定刚性并且能够控释内容物的材料如介孔二氧化硅、以聚乳酸-羟基乙酸共聚物为代表的阳离子高分子材料等来模拟细胞骨架的结构,在维持仿生外泌体形态结构、控制治疗性内容物的释放、维持生物膜的稳定性上具有一定的价值。这些人造外泌体由于具有类似于天然外泌体的粒径,可以减少其在肝脏、脾脏、肺脏中的滞留,良好的循环稳定性与较长的半衰期并且优异的生物相容性使其更容易被细胞摄取。以血小板膜为代表的生物膜具有靶向心肌梗死区域的作用。因此研究人员选择静脉注射这一相较于心肌内注射、冠脉内注射损伤更小的给药方式,发现在小鼠心肌梗死模型或心肌梗死缺血再灌注模型中这些人造外泌体可以很好地实现免疫逃逸、靶向受损心肌、治疗心肌梗死的预期功能。"


通过静脉注射给药虽然能提高在心肌梗死区域的药物浓度,但是药物在体内的各器官分布实验显示其在肝脏的富集是最多的[29],尽管生物毒性实验未发现这些药物对于肝脏的毒性,但是非目的器官的富集会造成药物的大量浪费,因此如何在未来的研究中进一步减少人造外泌体在除心脏外其他器官中的聚集提高人造外泌体的治疗效率是一个亟待解决的问题。同样的,全人造的仿生外泌体与生物膜融合的方式改造外泌体的手段同样存在如生物材料的来源、获取与存储与人工材料相比成本较高,且需要伦理学上的解释等问题。 2.3 人造外泌体的生物安全性评价 不论是天然来源的外泌体还是人造外泌体,由于其本身是细胞来源的具有活性,可以在生物体内自行降解且具有低免疫原性的纳米级囊泡,一般不会引发免疫或炎症反应,也正是这些特性使得外泌体在改善化学药物生物相容性方面优于其他载体[70]。脂质体、聚合物纳米颗粒和无机纳米颗粒等合成纳米载体在生物分布和生物利用度方面无法与外泌体相比[71-72]。外泌体是天然产生的,在体内半衰期更长,可以转移内源性生物分子,与脂质体和基于病毒的药物递送系统相比,外泌体被选为感兴趣和潜在的候选者[73]。无论是基于天然外泌体的改造还是模拟外泌体的仿生均保留了外泌体良好的生物活性及生物相容性。不论是在体外细胞毒性、活性实验,还是在体内实验中对实验动物肝脏、肾脏以及肺脏等重要器官中蓄积以及功能指标的研究发现,对外泌体的生物纳米改造几乎不会造成严重的生物安全性危害,但是对于这些生物制品在未来的生物安全性评价仍需多中心、长期的临床前及临床研究。"

| [1] THOMAS H, DIAMOND J, VIECO A, et al. Global Atlas of Cardiovascular Disease 2000-2016: the Path to Prevention and Control. Glob Heart. 2018;13(3):143-163. [2] 中国心血管健康与疾病报告编写组.中国心血管健康与疾病报告2020概要[J].中国循环杂志,2021,36(6):25. [3] JAMES SL, ABATE D, ABATE KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789-1858. [4] ONG SB, HERNANDEZ-RESENDIZ S, CRESPO-AVILAN GE, et al. Inflammation following acute myocardial infarction: multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacol Ther. 2018;186:73-87. [5] FRANGOGIANNIS NG. Regulation of the inflammatory response in cardiac repair. Circ Res. 2012;110(1):159-173. [6] GUO R, MORIMATSU M, FENG T, et al. Stem cell-derived cell sheet transplantation for heart tissue repair in myocardial infarction. Stem Cell Res Ther. 2020;11(1):19. [7] HONG KU, LI QH, GUO Y, et al. A highly sensitive and accurate method to quantify absolute numbers of c-kit+ cardiac stem cells following transplantation in mice. Basic Res Cardiol. 2013;108(3):346. [8] DUELEN R, SAMPAOLESI M. Stem cell technology in cardiac regeneration: a pluripotent stem cell promise. EBioMedicine. 2017;16:30-40. [9] STREMERSCH S, DE SMEDT SC, RAEMDONCK K. Therapeutic and diagnostic applications of extracellular vesicles. J Control Release. 2016;244(Pt B):167-183. [10] WANG X, ZHU Y, WU C, et al. Adipose-derived mesenchymal stem cells-derived exosomes carry microRNA-671 to alleviate myocardial infarction through inactivating the TGFBR2/Smad2 axis. Inflammation. 2021;44(5):1815-1830. [11] CHEN L, WANG Y, PAN Y, et al. Cardiac progenitor-derived exosomes protect ischemic myocardium from acute ischemia/reperfusion injury. Biochem Biophys Res Commun. 2013;431(3):566-571. [12] HUANG P, WANG L, LI Q, et al. Atorvastatin enhances the therapeutic efficacy of mesenchymal stem cells-derived exosomes in acute myocardial infarction via up-regulating long non-coding RNA H19. Cardiovasc Res. 2020;116(2):353-367. [13] ARSLAN F, LAI RC, SMEETS MB, et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013;10(3):301-312. [14] GAO L, WANG L, WEI Y, et al. Exosomes secreted by hiPSC-derived cardiac cells improve recovery from myocardial infarction in swine. Sci Transl Med. 2020; 12(561):eaay1318. [15] HUANG P, WANG L, LI Q, et al. Combinatorial treatment of acute myocardial infarction using stem cells and their derived exosomes resulted in improved heart performance. Stem Cell Res Ther. 2019;10(1):300. [16] KOOIJMANS SA, ALEZA CG, ROFFLER SR, et al. Display of GPI-anchored anti-EGFR nanobodies on extracellular vesicles promotes tumour cell targeting. J Extracell Vesicles. 2016;5:31053. [17] NAKASE I, NOGUCHI K, AOKI A, et al. Arginine-rich cell-penetrating peptide-modified extracellular vesicles for active macropinocytosis induction and efficient intracellular delivery. Sci Rep. 2017;7(1):1991. [18] PENG Q, MU H. The potential of protein-nanomaterial interaction for advanced drug delivery. J Control Release. 2016;225:121-132. [19] JANG SC, GHO YS. Could bioengineered exosome-mimetic nanovesicles be an efficient strategy for the delivery of chemotherapeutics? Nanomedicine (Lond). 2014;9(2):177-180. [20] JANG SC, KIM OY, YOON CM, et al. Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano. 2013;7(9):7698-7710. [21] NASIRI KENARI A, KASTANIEGAARD K, GREENING DW, et al. Proteomic and post-translational modification profiling of exosome-mimetic nanovesicles compared to exosomes. Proteomics. 2019;19(8):e1800161. [22] LUNAVAT TR, JANG SC, NILSSON L, et al. RNAi delivery by exosome-mimetic nanovesicles - Implications for targeting c-Myc in cancer. Biomaterials. 2016;102:231-238. [23] YANG Z, XIE J, ZHU J, et al. Functional exosome-mimic for delivery of siRNA to cancer: in vitro and in vivo evaluation. J Control Release. 2016;243:160-171. [24] GAO J, DONG X, WANG Z. Generation, purification and engineering of extracellular vesicles and their biomedical applications. Methods. 2020;177:114-125. [25] MENG W, HE C, HAO Y, et al. Prospects and challenges of extracellular vesicle-based drug delivery system:considering cell source. Drug Deliv. 2020;27(1):585-598. [26] LIU Y, WANG Y, LV Q, et al. Exosomes: from garbage bins to translational medicine. Int J Pharm. 2020;583:119333. [27] ZHUANG X, XIANG X, GRIZZLE W, et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther. 2011;19(10):1769-1779. [28] LEE Y, EL ANDALOUSSI S, WOOD MJ. Exosomes and microvesicles:extracellular vesicles for genetic information transfer and gene therapy. Hum Mol Genet. 2012;21(R1):R125-R134. [29] HU S, WANG X, LI Z, et al. Platelet membrane and stem cell exosome hybrid enhances cellular uptake and targeting to heart injury. Nano Today. 2021;39:101210. [30] XU X, LIANG Y, LI X, et al. Exosome-mediated delivery of kartogenin for chondrogenesis of synovial fluid-derived mesenchymal stem cells and cartilage regeneration. Biomaterials. 2021;269:120539. [31] DUAN L, XU L, XU X, et al. Exosome-mediated delivery of gene vectors for gene therapy. Nanoscale. 2021;13(3):1387-1397. [32] WEI W, AO Q, WANG X, et al. Mesenchymal stem cell-derived exosomes: a promising biological tool in nanomedicine. Front Pharmacol. 2020;11:590470. [33] TSIAPALIS D, O’DRISCOLL L. Mesenchymal stem cell derived extracellular vesicles for tissue engineering and regenerative medicine applications. Cells. 2020;9(4):991. [34] MAN K, BRUNET M Y, JONES M C, et al. Engineered extracellular vesicles:tailored-made nanomaterials for medical applications. Nanomaterials (Basel). 2020;10(9):1838. [35] MENTKOWSKI KI, TARVIRDIZADEH T, MANZANERO CA, et al. Surface engineering enhances the therapeutic potential of extracellular vesicles following acute myocardial infarction. BioRxiv. 2022. doi: /10.1101/2022.01.31.478512. [36] MENTKOWSKI KI, LANG JK. Exosomes engineered to express a cardiomyocyte binding peptide demonstrate improved cardiac retention in vivo. Sci Rep. 2019; 9(1):10041. [37] VANDERGRIFF A, HUANG K, SHEN D, et al. Targeting regenerative exosomes to myocardial infarction using cardiac homing peptide. Theranostics. 2018;8(7):1869-1878. [38] CIULLO A, BIEMMI V, MILANO G, et al. Exosomal expression of CXCR4 targets cardioprotective vesicles to myocardial infarction and improves outcome after systemic administration. Int J Mol Sci. 2019;20(3):468. [39] WANG X, CHEN Y, ZHAO Z, et al. Engineered exosomes with ischemic myocardium-targeting peptide for targeted therapy in myocardial infarction. J Am Heart Assoc. 2018;7(15):e008737. [40] KANKI S, JAALOUK DE, LEE S, et al. Identification of targeting peptides for ischemic myocardium by in vivo phage display. J Mol Cell Cardiol. 2011;50(5):841-848. [41] ANTES J, MIDDLETON RC, LUTHER KM, et al. Targeting extracellular vesicles to injured tissue using membrane cloaking and surface display. J Nanobiotechnology. 2018;16(1):61. [42] KIM H, YUN N, MUN D, et al. Cardiac-specific delivery by cardiac tissue-targeting peptide-expressing exosomes. Biochem Biophys Res Commun. 2018;499(4):803-808. [43] ZHU LP, TIAN T, WANG JY, et al. Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics. 2018;8(22):6163-6177. [44] LI Q, HUANG Z, WANG Q, et al. Targeted immunomodulation therapy for cardiac repair by platelet membrane engineering extracellular vesicles via hitching peripheral monocytes. Biomaterials. 2022;284:121529. [45] LI Q, SONG Y, WANG Q, et al. Engineering extracellular vesicles with platelet membranes fusion enhanced targeted therapeutic angiogenesis in a mouse model of myocardial ischemia reperfusion. Theranostics. 2021;11(8):3916-3931. [46] ZHANG N, SONG Y, HUANG Z, et al. Monocyte mimics improve mesenchymal stem cell-derived extracellular vesicle homing in a mouse MI/RI model. Biomaterials. 2020;255:120168. [47] FANG R H, KROLL A V, GAO W, et al. Cell membrane coating nanotechnology. Adv Mater. 2018;30(23):e1706759. [48] SUN J, MUZ B, ALHALLAK K, et al. Targeting CD47 as a Novel Immunotherapy for Multiple Myeloma. Cancers (Basel). 2020;12(2):305. [49] BARKAL AA, BREWER RE, MARKOVIC M, et al. CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy. Nature. 2019;572(7769):392-396. [50] FANG RH, JIANG Y, FANG JC, et al. Cell membrane-derived nanomaterials for biomedical applications. Biomaterials. 2017;128:69-83. [51] LI H, JIN K, LUO M, et al. Size dependency of circulation and biodistribution of biomimetic nanoparticles: red blood cell membrane-coated nanoparticles. Cells. 2019;8(8):881. [52] BEN-AKIVA E, MEYER RA, YU H, et al. Biomimetic anisotropic polymeric nanoparticles coated with red blood cell membranes for enhanced circulation and toxin removal. Sci Adv. 2020;6(16):eaay9035. [53] HU CM, FANG RH, WANG KC, et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature. 2015;526(7571):118-121. [54] WANG D, WANG S, ZHOU Z, et al. White blood cell membrane-coated nanoparticles: recent development and medical applications. Adv Healthc Mater. 2022;11(7):e2101349. [55] YAO C, WU W, TANG H, et al. Self-assembly of stem cell membrane-camouflaged nanocomplex for microRNA-mediated repair of myocardial infarction injury. Biomaterials. 2020;257:120256. [56] FANG RH, HU CM, LUK BT, et al. Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano Lett. 2014;14(4):2181-2188. [57] MODERY-PAWLOWSKI CL, KUO HH, BALDWIN WM, et al. A platelet-inspired paradigm for nanomedicine targeted to multiple diseases. Nanomedicine (Lond). 2013;8(10):1709-1727. [58] LEE JR, PARK BW, KIM J, et al. Nanovesicles derived from iron oxide nanoparticles-incorporated mesenchymal stem cells for cardiac repair. Sci Adv. 2020;6(18):eaaz0952. [59] LIU S, CHEN X, BAO L, et al. Treatment of infarcted heart tissue via the capture and local delivery of circulating exosomes through antibody-conjugated magnetic nanoparticles. Nat Biomed Eng. 2020;4(11):1063-1075. [60] ZHANG X, ZHANG Y, ZHANG R, et al. Biomimetic design of artificial hybrid nanocells for boosted vascular regeneration in ischemic tissues. Adv Mater. 2022; 34(14):e2110352. [61] CHEN J, SONG Y, WANG Q, et al. Targeted neutrophil-mimetic liposomes promote cardiac repair by adsorbing proinflammatory cytokines and regulating the immune microenvironment. J Nanobiotechnology. 2022;20(1):218. [62] BAO L, DOU G, TIAN R, et al. Engineered neutrophil apoptotic bodies ameliorate myocardial infarction by promoting macrophage efferocytosis and inflammation resolution. Bioact Mater. 2022;9:183-197. [63] XU L, CHEN Y, JIN Q, et al. Biomimetic PLGA microbubbles coated with platelet membranes for early detection of myocardial ischaemia-reperfusion injury. Mol Pharm. 2021;18(8):2974-2985. [64] XUE Y, ZENG G, CHENG J, et al. Engineered macrophage membrane-enveloped nanomedicine for ameliorating myocardial infarction in a mouse model. Bioeng Transl Med. 2021;6(2):e10197. [65] ZHANG R, LUO W, ZHANG Y, et al. Particle-based artificial three-dimensional stem cell spheroids for revascularization of ischemic diseases. Sci Adv. 2020; 6(19):eaaz8011. [66] SU T, HUANG K, MA H, et al. Platelet-inspired nanocells for targeted heart repair after ischemia/reperfusion injury. Adv Funct Mater. 2019;29(4):1803567. [67] KOOIJMANS SA, VADER P, VAN DOMMELEN SM, et al. Exosome mimetics: a novel class of drug delivery systems. Int J Nanomedicine. 2012;7:1525-1541. [68] ZANINETTI C, SACHS L, PALANKAR R. Role of platelet cytoskeleton in platelet biomechanics: current and emerging methodologies and their potential relevance for the investigation of inherited platelet disorders. Hamostaseologie. 2020;40(3):337-347. [69] GAO J, SONG Y, WANG Q, et al. Precisely co-delivery of protein and ROS scavenger with platesomes for enhanced endothelial barrier preservation against myocardial ischemia reperfusion injury. Chem Eng J. 2022;44(2):446. [70] WANG J, DONG Y, LI Y, et al. Designer exosomes for active targeted chemo-photothermal synergistic tumor therapy. Adv Funct Mater. 2018;28(18):1707360. [71] BUNGGULAWA EJ, WANG W, YIN T, et al. Recent advancements in the use of exosomes as drug delivery systems. J Nanobiotechnology. 2018;16(1):81. [72] ARYANI A, DENECKE B. Exosomes as a nanodelivery system: a key to the future of neuromedicine? Mol Neurobiol. 2016;53(2):818-834. [73] MURALIDHARAN-CHARI V, CLANCY JW, SEDGWICK A, et al. Microvesicles: mediators of extracellular communication during cancer progression. J Cell Sci. 2010;123(Pt 10):1603-1611. |
| [1] | Feng Ruiqin, Han Na, Zhang Meng, Gu Xinyi, Zhang Fengshi. Combination of 1% platelet-rich plasma and bone marrow mesenchymal stem cells improves the recovery of peripheral nerve injury [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 985-992. |
| [2] | Wang Wen, Zheng Pengpeng, Meng Haohao, Liu Hao, Yuan Changyong. Overexpression of Sema3A promotes osteogenic differentiation of dental pulp stem cells and MC3T3-E1 [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 993-999. |
| [3] | Qiu Xiaoyan, Li Bixin, Li Jingdi, Fan Chuiqin, Ma Lian, Wang Hongwu. Differentiation of insulin-producing cells from human umbilical cord mesenchymal stem cells infected by MAFA-PDX1 overexpressed lentivirus [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1000-1006. |
| [4] | Liu Qiwei, Zhang Junhui, Yang Yuan, Wang Jinjuan. Role and mechanism of umbilical cord mesenchymal stem cells on polycystic ovary syndrome [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1015-1020. |
| [5] | Liu Jianhong, Liao Shijie, Li Boxiang, Tang Shengping, Wei Zhendi, Ding Xiaofei. Extracellular vesicles carrying non-coding RNA regulate the activation of osteoclasts [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1076-1082. |
| [6] | Wang Shanshan, Shu Qing, Tian Jun. Physical factors promote osteogenic differentiation of stem cells [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1083-1090. |
| [7] | Pan Xiaolong, Fan Feiyan, Ying Chunmiao, Liu Feixiang, Zhang Yunke. Effect and mechanism of traditional Chinese medicine on inhibiting the aging of mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1091-1098. |
| [8] | Xu Canli, He Wenxing, Wang Lei, Wu Fangting, Wang Jiahui, Duan Xuelin, Zhao Tiejian, Zhao Bin, Zheng Yang. Bibliometric analysis of researches on liver organoids [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1099-1104. |
| [9] | Zhuge Xiaoxuan, Li Ce, Bao Guangjie, Kang Hong. Potential value of canonical and non-canonical roles of connexin 43 in disease treatment [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1130-1136. |
| [10] | Ma Shuwei, He Sheng, Han Bing, Zhang Liaoyun. Exosomes derived from mesenchymal stem cells in treatment of animals with acute liver failure: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1137-1142. |
| [11] | Sun Yukang, Song Lijuan, Wen Chunli, Ding Zhibin, Tian Hao, Ma Dong, Ma Cungen, Zhai Xiaoyan. Visualization analysis of stem cell therapy for myocardial infarction based on Web of Science in recent ten years [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1143-1148. |
| [12] | Zhang Kefan, Shi Hui. Research status and application prospect of cytokine therapy for osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(6): 961-967. |
| [13] | Wei Yuanxun, Chen Feng, Lin Zonghan, Zhang Chi, Pan Chengzhen, Wei Zongbo. The mechanism of Notch signaling pathway in osteoporosis and its prevention and treatment with traditional Chinese medicine [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 587-593. |
| [14] | Lin Feng, Cheng Ling, Gao Yong, Zhou Jianye, Shang Qingqing. Hyaluronic acid hydrogel-encapsulated bone marrow mesenchymal stem cells promote cardiac function in myocardial infarction rats (III) [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(3): 355-359. |
| [15] | Bi Yujie, Ma Dujun, Peng Liping, Zhou Ziqiong, Zhao Jing, Zhu Houjun, Zhong Qiuhui, Yang Yuxin. Strategy and significance of Chinese medicine combined with medical hydrogel for disease treatment [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(3): 419-425. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||