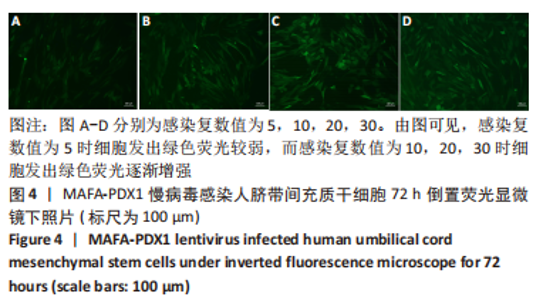
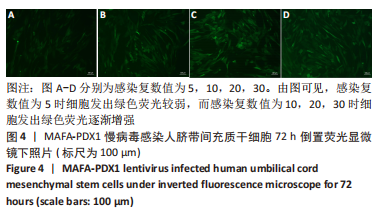
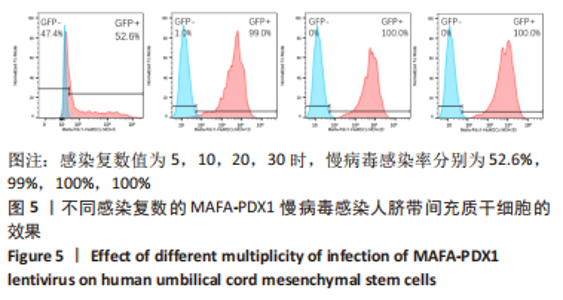
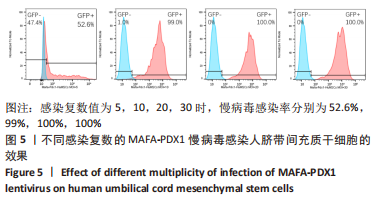
Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (7): 1000-1006.doi: 10.12307/2024.104
Previous Articles Next Articles
Differentiation of insulin-producing cells from human umbilical cord mesenchymal stem cells infected by MAFA-PDX1 overexpressed lentivirus
Qiu Xiaoyan1, Li Bixin1, Li Jingdi1, Fan Chuiqin1, Ma Lian1, 2, 3, Wang Hongwu1, 4
- 1Department of Pediatrics, The Second Affiliated Hospital of Shantou University Medical College, Shantou 515041, Guangdong Province, China; 2Department of Pediatrics Research Institute, Shenzhen Children’s Hospital, Shenzhen 518038, Guangdong Province, China; 3The Third Affiliated Hospital of Guangzhou Medical University (Women’s and Children’s Hospital of Guangzhou Medical University), Guangzhou 512000, Guangdong Province, China;
-
Received:2023-01-07Accepted:2023-03-06Online:2024-03-08Published:2023-07-15 -
Contact:Wang Hongwu, MD, Chief physician, Department of Pediatrics, The Second Affiliated Hospital of Shantou University Medical College, Shantou 515041, Guangdong Province, China; Stem Cell Research Center of Shantou University Medical College, Shantou 515041, Guangdong Province, China -
About author:Qiu Xiaoyan, Master, Attending physician, Department of Pediatrics, The Second Affiliated Hospital of Shantou University Medical College, Shantou 515041, Guangdong Province, China -
Supported by:2020 Guangdong Science and Technology Plan Major Project, No. SFK[2020]53-112 (to WHW)
CLC Number:
Cite this article
Qiu Xiaoyan, Li Bixin, Li Jingdi, Fan Chuiqin, Ma Lian, Wang Hongwu. Differentiation of insulin-producing cells from human umbilical cord mesenchymal stem cells infected by MAFA-PDX1 overexpressed lentivirus[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1000-1006.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
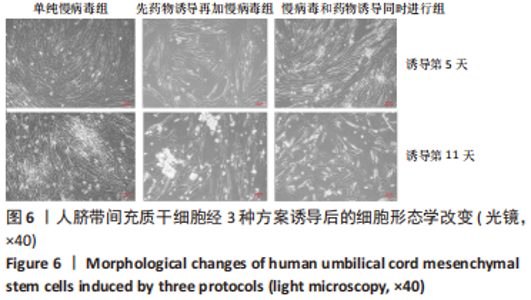
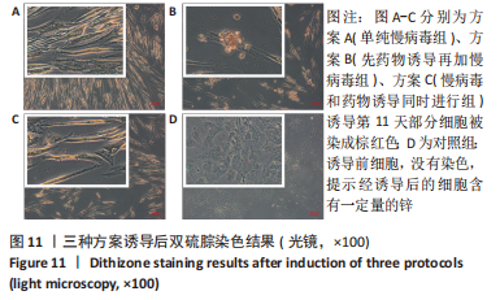
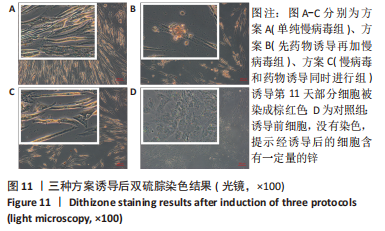
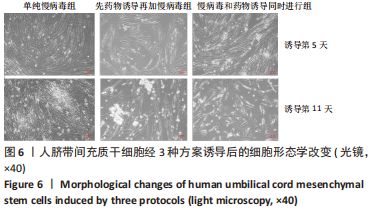
2.5 MAFA-PDX1修饰的HuMSCs向胰岛素分泌细胞分化 2.5.1 细胞形态学观察 见图6。①方案A(单纯慢病毒组):诱导第5天,细胞形态失去明显成纤维结构,细胞呈圆形或者类圆形,体积略增大,折光性增强,并开始出现细胞聚集;诱导第11天,部分细胞聚集成团,并呈半悬浮状态。无论是第5天还是第11天,方案A诱导分化后聚集成团细胞数量均少于方案B和方案C。②方案B(先药物诱导再加慢病毒组):诱导第5天,部分细胞呈圆形或多角形,周边突出逐渐缩短,部分细胞聚集成团,但成团状细胞数量较少,半悬浮生长;第11天细胞聚集生长更为明显,出现聚集生长的胰岛样细胞团。方案B第11天聚集成团的细胞数量明显多于其余2种诱导方案。③方案C(慢病毒和药物诱导同时进行组):诱导第5天,部分细胞呈圆形或多角形,周边突出逐渐缩短,部分细胞聚集成团,半悬浮生长,聚集成团的细胞较方案A和方案B多;诱导第11天细胞生长缓慢,细胞聚集成团数量较第5天减少。"
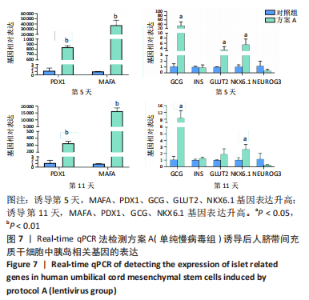
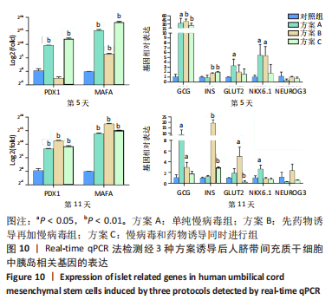
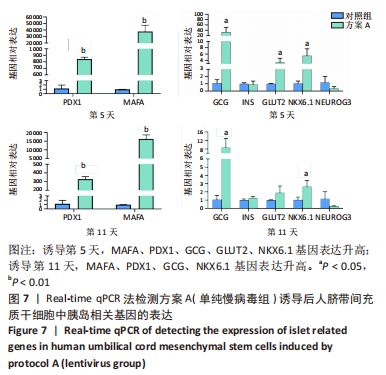
2.5.2 不同方案诱导后细胞中胰岛相关基因表达 (1)方案A(单纯慢病毒组)诱导后细胞中胰岛相关基因表达,见图7。诱导第5天时,与对照组相比,MAFA和PDX1基因表达显著升高,二者的相对表达量分别为37 989.71±7 685.54(P=0.002),844.09±22.49(P < 0.001);GCG、GLUT2、NKX6.1基因表达升高,其相对表达量分别为33.30±14.50(P=0.035),3.35±1.07(P=0.037),5.48±1.79 (P=0.026);INS和NEUROG3基因表达在统计学上无明显差异,分别为0.90±0.39(P=0.735),0.35±0.21(P=0.181)。诱导第11天时,与对照组相比,MAFA和PDX1基因表达显著升高,二者的相对表达量分别为16 307.57±1 890.56(P < 0.001),327.89±23.25(P < 0.001);GCG、NKX6.1基因表达升高,其相对表达量分别为8.69±3.47(P=0.037),2.65±0.59(P=0.025);INS、GLUT2和NEUROG3基因表达在统计学上无明显差异,分别为1.26±0.14(P=0.118),1.91±0.66(P=0.124),0.28±0.03 (P=0.139)。"
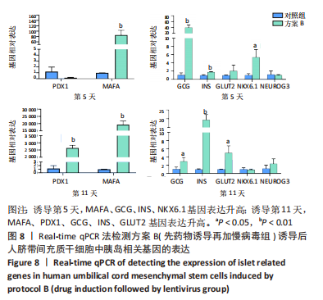
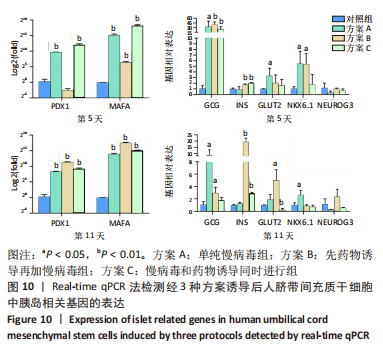
(2)方案B(先药物诱导再加慢病毒组)诱导后细胞中胰岛相关基因表达,见图8。诱导第5天时,与对照组相比,MAFA、GCG、INS、NKX6.1基因表达升高,其相对表达量分别为85.73±14.23(P=0.001),40.46±6.54(P=0.001),1.77±0.10(P=0.002),5.39±1.51(P=0.016);PDX1、GLUT2、NEUROG3基因表达在统计学上无明显差异,分别为0.18±0.06 (P=0.084),2.06±1.16(P=0.269),1.03±0.10(P=0.772)。诱导第11天时,与对照组相比,MAFA和PDX1基因表达显著升高,二者的相对表达量分别为193 881.49±20 119.50(P < 0.001),2 644.85±146.88(P < 0.001);GCG、INS、GLUT2基因表达升高,其相对表达量分别为2.98±0.73(P=0.033),19.40± 2.44(P < 0.001),5.02±1.38(P=0.015);NKX6.1、NEUROG3基因表达在统计学上无明显差异,分别为0.90±0.13(P=0.546),2.38±0.96(P=0.226)。MAFA、GCG、INS基因在诱导第5,11天均表达升高,其中MAFA和INS基因第11天表达量显著高于第5天,GCG基因第5天表达量高于第11天。NKX6.1基因在诱导第5天升高,第11天无明显表达。"

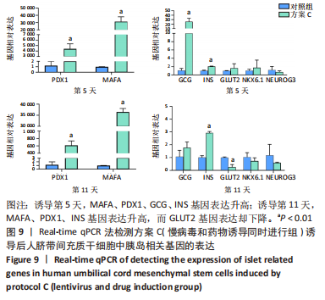
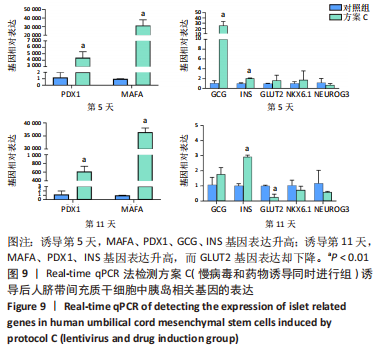
(3)方案C(慢病毒和药物诱导同时进行组)诱导后细胞中胰岛相关基因表达,见图9。诱导第5天时,与对照组相比,MAFA和PDX1基因表达显著升高,二者的相对表达量分别为314 696.50±52 937.80(P=0.001),4 414.68±736.09 (P=0.001);GCG、INS基因表达升高,其相对表达量分别为25.53±6.23(P=0.005),2.07±0.04(P < 0.001);GLUT2、NKX6.1、NEUROG3基因表达在统计学上无明显差异,分别1.64±0.85(P=0.350),1.77±1.46(P=0.523),0.70±0.25 (P=0.406)。诱导第11天时,与对照组相比,MAFA和PDX1基因表达显著升高,二者的相对表达量分别为36 448.73±1 309.93(P < 0.001),617.14±95.67(P=0.001);INS基因表达升高,其相对表达量为2.93±0.09(P < 0.001);GLUT2基因表达降低,其相对表达量为0.26±0.16(P=0.003);GCG、NKX6.1、NEUROG3基因表达在统计学上无明显差异,分别为1.78±0.35(P=0.135),0.74±0.20(P=0.272),0.59±0.03(P=0.297)。MAFA、PDX1、INS基因在诱导第5,11天均表达升高,其中MAFA、PDX1基因第5天表达量高于第11天,INS基因第11天表达量高于第5天。GCG在诱导第5天表达升高,第11天无明显表达。NKX6.1基因在第5,11天均无明显表达。"
| [1] PUNTHAKEE Z, GOLDENBERG R, KATZ P. Definition, Classification and Diagnosis of Diabetes,Prediabetes and Metabolic Syndrome. Can J Diabetes. 2018;42 Suppl 1:S10-S15. [2] HARREITER J, RODEN M. Diabetes mellitus-Definition,classification, diagnosis, screening and prevention (Update 2019). Wien Klin Wochenschr. 2019;131(Suppl 1):6-15. [3] HELMAN A, MELTON DA. A Stem Cell Approach to Cure Type 1 Diabetes. Cold Spring Harb Perspect Biol. 2021;13(1):a035741. [4] QUISKAMP N, BRUIN JE, KIEFFER TJ. Differentiation of human pluripotent stem cells into β-cells: Potential and challenges. Best Pract Res Clin Endocrinol Metab. 2015;29(6):833-847. [5] DING DC, CHANG YH, SHYU WC, et al. Human umbilical cord mesenchymal stem cells: a new era for stem cell therapy. Cell Transplant. 2015;24(3):339-347. [6] XIE Q, LIU R, JIANG J, et al. What is the impact of human umbilical cord mesenchymal stem cell transplantation on clinical treatment? Stem Cell Res Ther. 2020;11(1):519. [7] LI T, XIA M, GAO Y, et al. Human umbilical cord mesenchymal stem cells: an overview of their potential in cell-based therapy. Expert Opin BiolTher. 2015; 15(9):1293-306. [8] BI S, NIE Q, WANG WQ, et al. Human Umbilical Cord Mesenchymal Stem Cells Therapy for Insulin Resistance: A Novel Strategy in Clinical Implication. Curr Stem Cell Res Ther. 2018;13(8):658-664. [9] XIAO X, GUO P, SHIOTA C, et al. Endogenous Reprogramming of Alpha Cells into Beta Cells, Induced by Viral Gene Therapy,Reverses Autoimmune Diabetes. Cell Stem Cell. 2018;22(1):78-90.e4. [10] ZHU Y, LIU Q, ZHOU Z, et al. PDX1, Neurogenin-3, and MAFA: critical transcription regulators for beta cell development and regeneration. Stem Cell Res Ther. 2017;8(1):240. [11] ANGUELA XM, HIGH KA. Entering the Modern Era of Gene Therapy. Annu Rev Med. 2019;70:273-288. [12] MILONE MC, O’DOHERTY U. Clinical use of lentiviral vectors. Leukemia. 2018; 32(7):1529-1541. [13] WANG X, MA C, RODRÍGUEZ LABRADA R, et al. Recent advances in lentiviral vectors for gene therapy. Sci China Life Sci. 2021;64(11):1842-1857. [14] MUNIS AM. Gene Therapy Applications of Non-Human Lentiviral Vectors. Viruses. 2020;12(10):1106. [15] AKINCI E, BANGA A, GREDER LV, et al. Reprogramming of pancreatic exocrine cells towards a beta (β) cell character using Pdx1, Ngn3 and MafA. Biochem J. 2012;442(3):539-550. [16] JENNINGS RE, BERRY AA, STRUTT JP, et al. Human pancreas development. Development. 2015;142(18):3126-3137. [17] JENNINGS RE, BERRY AA, KIRKWOOD-WILSON R, et al. Development of the human pancreas from foregut to endocrine commitment. Diabetes. 2013;62(10):3514-3522. [18] HENRY BM, SKINNINGSRUD B, SAGANIAK K, et al. Tomaszewski KA. Development of the human pancreas and its vasculature - An integrated review covering anatomical, embryological, histological, and molecular aspects. Ann Anat. 2019; 221:115-124. [19] PETERSEN MBK, GONÇALVES CAC, KIM YH, et al. Recapitulating and Deciphering Human Pancreas Development From Human Pluripotent Stem Cells in a Dish. Curr Top Dev Biol. 2018;129:143-190. [20] RUBIO-CABEZAS O, JENSEN JN, HODGSON MI, et al. Permanent Neonatal Diabetes and Enteric Anendocrinosis Associated With Biallelic Mutations in NEUROG3. Diabetes. 2011;60(4):1349-1353. [21] SCHREIBER V, MERCIER R, JIMÉNEZ S, et al. Extensive NEUROG3 occupancy in the human pancreatic endocrine gene regulatory network. Mol Metab. 2021; 53:101313. [22] SOLORZANO-VARGAS RS, BJERKNES M, WANG J, et al. Null mutations of NEUROG3 are associated with delayed-onset diabetes mellitus. JCI Insight. 2020; 5(1):e127657. [23] MCGRATH PS, WATSON CL, INGRAM C, et al. The Basic Helix-Loop-Helix Transcription Factor NEUROG3 Is Required for Development of the Human Endocrine Pancreas. Diabetes. 2015;64(7):2497-2505. [24] YONG HJ, XIE G, LIU C, et al. Gene Signatures of NEUROGENIN3+ Endocrine Progenitor Cells in the Human Pancreas. Front Endocrinol(Lausanne). 2021;12: 736286. [25] LIU J, BANERJEE A, HERRING CA, et al. Neurog3-Independent Methylation Is the Earliest Detectable Mark Distinguishing Pancreatic Progenitor Identity. Dev Cell. 2019;48(1):49-63.e7. [26] VERES A, FAUST AL, BUSHNELL HL, et al. Charting cellular identity during human in vitro β-cell differentiation. Nature. 2019;569(7756):368-373. [27] ITOH M, TAKIZAWA Y, HANAI S, et al. Partial loss of pancreas endocrine and exocrine cells of human ARX-null mutation: consideration of pancreas differentiation. Differentiation. 2010;80(2-3):118-122. [28] GAGE BK, ASADI A, BAKER RK, et al. The Role of ARX in Human Pancreatic Endocrine Specification. PLoS One. 2015;10(12):e0144100. [29] RIEDEL MJ, ASADI A, WANG R, et al. Immunohistochemical characterisation of cells co-producing insulin and glucagon in the developing human pancreas. Diabetologia. 2012;55(2):372-381. |
| [1] | Liu Qiwei, Zhang Junhui, Yang Yuan, Wang Jinjuan. Role and mechanism of umbilical cord mesenchymal stem cells on polycystic ovary syndrome [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1015-1020. |
| [2] | Wang Liping, Lian Tianxing, Hu Yongrong, Yang Hongsheng, Zeng Zhimou, Liu Hao, Qu Bo. HU value of chest CT vertebral body in the opportunistic screening of type 2 diabetes mellitus osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(6): 950-954. |
| [3] | Xue Jingwen, Wang Fangfang, Zhang Xin, Pang Ruifeng, Wang Xiaoye, Ma Xiaoru. Effect of ganoderma spore on mitochondrial autophagy and apoptosis in testicular tissue of diabetic rats [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 562-568. |
| [4] | Zheng Mingkui, Xue Chenhui, Guan Xiaoming, Ma Xun. Human umbilical cord mesenchymal stem cell-derived exosomes reduce the permeability of blood-spinal cord barrier after spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(1): 50-55. |
| [5] | Wang Ji, Zhang Min, Yang Zhongya, Zhang Long. A review of physical activity intervention in type 2 diabetes mellitus with sarcopenia [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1272-1277. |
| [6] | Sun Jiajia, Zhu Haidi, Lu Yun, Zhang Kai. Comparison of bone metabolism markers between type 2 diabetes mellitus and non-type 2 diabetes mellitus patients with hip fracture [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1156-1160. |
| [7] | Cui Lianxu, Jiang Wenkang, Lu Dahong, Xu Junrong, Liu Xiaocui, Wang Bingyun. Clinical-grade human umbilical cord mesenchymal stem cells affect the improvement of neurological function in rats with traumatic brain injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 835-839. |
| [8] | Qian Guang, Yu Yueming, Dong Youhai, Hong Yang, Wang Minghai. Serum pentosidine level and trabecular bone score affect the severity of vertebral fractures in type 2 diabetes patients [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(36): 5870-5874. |
| [9] | Leng Siyi, Pu Rui, Chen Ziyang, Yang Qihang, Song Yongjing, Liu Hui. Roles of exercise intervention in intestinal flora in autoimmune diseases [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(32): 5219-5226. |
| [10] | Liu Chunli, Yan Yujuan, Mo Liwen, Wu Zhijie, Zhang Li. Effects of puerarin on osteoclast differentiation of RAW264.7 cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(32): 5114-5119. |
| [11] | Gu Peng, Pu Bin, Chen Junbang, Yue Dan, Xin Qiao, Zeng Zhanpeng, Zheng Xiaohui. Correlation between new sarcopenia index and bone mineral density in postmenopausal patients with type 2 diabetes mellitus [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(31): 5009-5014. |
| [12] | Chen Zili, Cao Ning, Xu Meng, Jiang Yan, Ji Meichao, Zheng Yangyang, Yang Lili. Biological characteristics of tumor necrosis factor-alpha-primed human umbilical cord mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(24): 3780-3787. |
| [13] | Wu Shuangshuang, Zhang Ying, Kou Xianjuan. Treadmill exercise improves cognitive dysfunction in diabetic rats [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(2): 208-215. |
| [14] | Zhang Yuwei, Liu Chuanchuan, Mao Jiaqi, Zhang Qingqing, Liu Hong, Chen Ying, Ma Lan. Umbilical cord mesenchymal stem cell-derived exosomes treated with hypoxic preconditioning inhibits proliferation of pulmonary artery smooth muscle cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(19): 2986-2992. |
| [15] | Xu Huifang, Shan Ping, Li Lijuan. Obtaining microglia-like cells after mouse corneal epithelial cell induction [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(19): 3017-3022. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||