Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (4): 562-568.doi: 10.12307/2023.969
Previous Articles Next Articles
Effect of ganoderma spore on mitochondrial autophagy and apoptosis in testicular tissue of diabetic rats
Xue Jingwen1, 2, Wang Fangfang1, 2, Zhang Xin1, Pang Ruifeng1, Wang Xiaoye1, Ma Xiaoru1, 2
- 1School of Basic Medicine, Jiamusi University, Jiamusi 154007, Heilongjiang Province, China; 2Key Laboratory of Microecology-Immunomodulatory Network and Related Diseases, School of Basic Medicine, Jiamusi University, Jiamusi 154007, Heilongjiang Province, China
-
Received:2022-11-21Accepted:2023-01-05Online:2024-02-08Published:2023-07-14 -
Contact:Ma Xiaoru, Master, Professor, School of Basic Medicine, Jiamusi University, Jiamusi 154007, Heilongjiang Province, China; Key Laboratory of Microecology-Immunomodulatory Network and Related Diseases, School of Basic Medicine, Jiamusi University, Jiamusi 154007, Heilongjiang Province, China -
About author:Xue Jingwen, Master candidate, School of Basic Medicine, Jiamusi University, Jiamusi 154007, Heilongjiang Province, China -
Supported by:Scientific Research Project of Higher Education Institutions of Heilongjiang Province, No. 2021-KYYWF-0593 (to WFF)
CLC Number:
Cite this article
Xue Jingwen, Wang Fangfang, Zhang Xin, Pang Ruifeng, Wang Xiaoye, Ma Xiaoru. Effect of ganoderma spore on mitochondrial autophagy and apoptosis in testicular tissue of diabetic rats[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 562-568.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
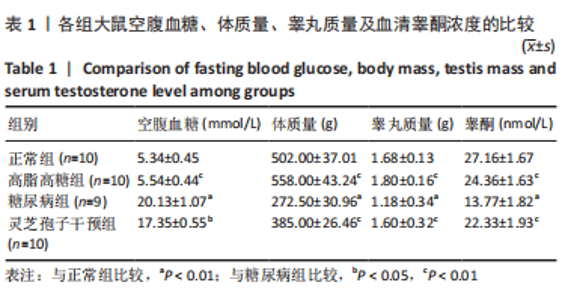
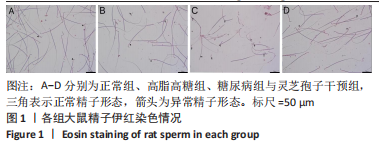
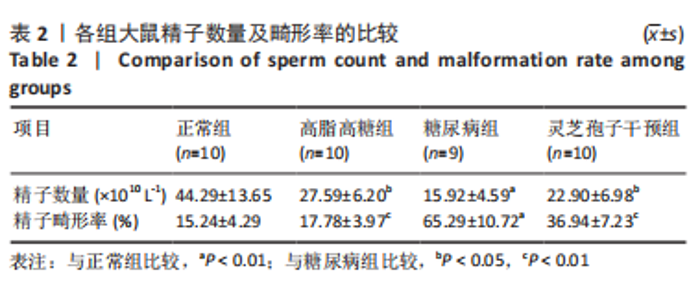
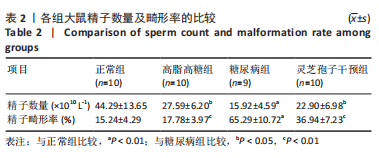
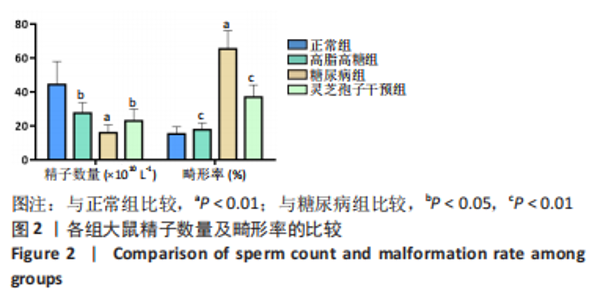
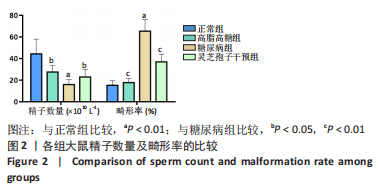
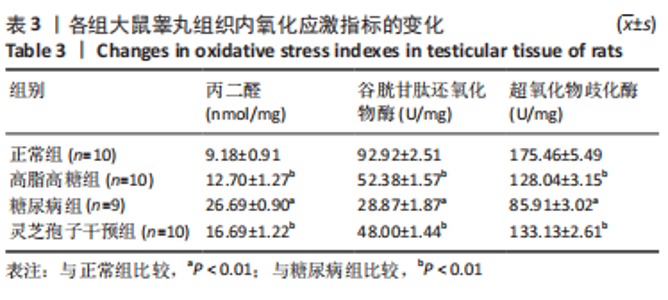
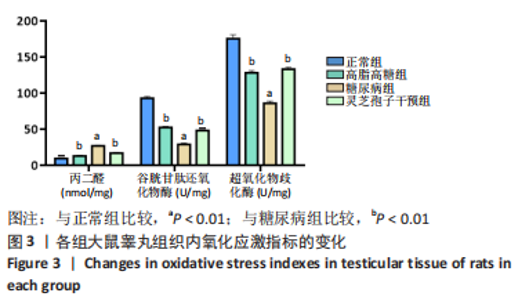
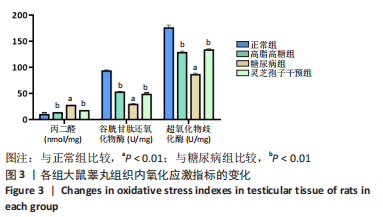
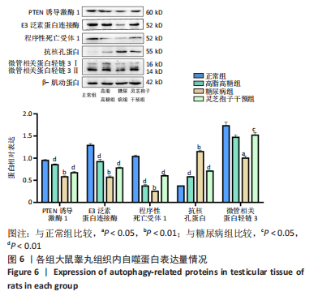
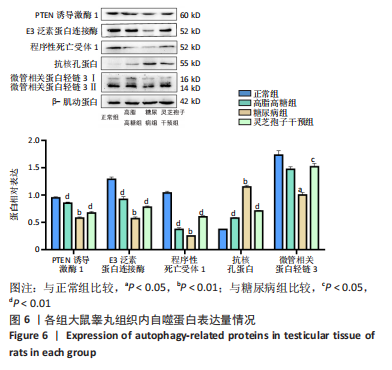
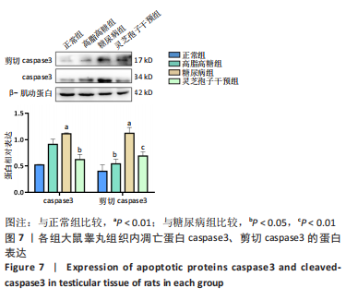
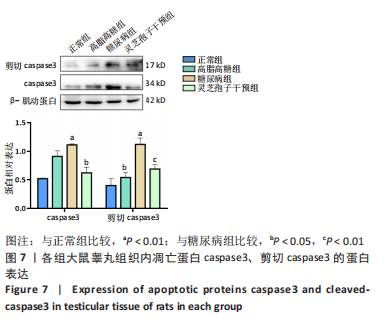
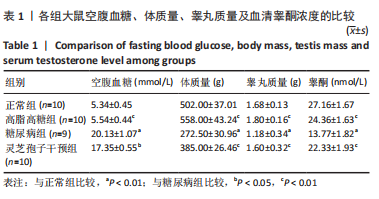
2.1 实验动物数量分析 实验选用40只雄性SD大鼠,每组大鼠10只。模型复制期间,糖尿病组有1只大鼠死亡,可能是由于腹腔注射时刺破其他器官造成死亡,最终39只大鼠进入结果分析。 2.2 各组大鼠血糖、体质量、睾丸质量及血清睾酮浓度检测结果 如表1所示,正常组和高脂高糖组大鼠体质量随饲养周期延长而增加,两组血糖水平无明显差异(P > 0.05);与正常组和高脂高糖组相比,糖尿病组大鼠血糖上升(P < 0.01),体质量和睾丸质量减少(P < 0.01),血清睾酮浓度下降(P < 0.01);与糖尿病组相比,灵芝孢子干预组大鼠血糖明显降低(P < 0.05),体质量和睾丸质量增加(P < 0.01),血清睾酮浓度显著上升(P < 0.01)。"
| [1] MEYHÖFER S, SCHMID SM. Diabeteskomplikationen-Diabetes und Nervensystem [Diabetes complications - diabetes and the nervous system]. Dtsch Med Wochenschr. 2020;145(22):1599-1605. [2] 杨知慧,孙立丽,任晓亮.2型糖尿病危险因素研究进展[J].实用糖尿病杂志,2020,16(6): 83-84. [3] HUANG J, YE Y, XIAO Y, et al. Geniposide ameliorates glucocorticoid-induced osteoblast apoptosis by activating autophagy. Biomed Pharmacother. 2022;155:113829. [4] KHALIL ASM, GIRIBABU N, YELUMALAI S, et al. Myristic acid defends against testicular oxidative stress, inflammation, apoptosis: Restoration of spermatogenesis, steroidogenesis in diabetic rats. Life Sci. 2021;278: 119605. [5] YUN HR, JO YH, KIM J, et al. Roles of Autophagy in Oxidative Stress. Int J Mol Sci. 2020;21(9):3289. [6] HAN YC, TANG SQ, LIU YT, et al. AMPK agonist alleviate renal tubulointerstitial fibrosis via activating mitophagy in high fat and streptozotocin induced diabetic mice. Cell Death Dis. 2021;12(10):925. [7] TONG M, SAITO T, ZHAI P, et al. Mitophagy Is Essential for Maintaining Cardiac Function During High Fat Diet-Induced Diabetic Cardiomyopathy. Circ Res. 2019;124(9):1360-1371. [8] ANSARI MY, KHAN NM, AHMAD I, et al. Parkin clearance of dysfunctional mitochondria regulates ROS levels and increases survival of human chondrocytes. Osteoarthritis Cartilage. 2018;26(8):1087-1097. [9] YAMASHITA A, MATSUOKA Y, MATSUDA M, et al. Dysregulation of p53 and Parkin Induce Mitochondrial Dysfunction and Leads to the Diabetic Neuropathic Pain. Neuroscience. 2019;416:9-19. [10] SU L, LI D, SU J, et al. Polysaccharides of Sporoderm-Broken Spore of Ganoderma lucidum Modulate Adaptive Immune Function via Gut Microbiota Regulation. Evid Based Complement Alternat Med. 2021;2021:8842062. [11] FANG L, ZHAO Q, GUO C, et al. Removing the sporoderm from the sporoderm-broken spores of Ganoderma lucidum improves the anticancer and immune-regulatory activity of the water-soluble polysaccharide. Front Nutr. 2022;9:1006127. [12] SHAHER F, WANG S, QIU H, et al. Effect and Mechanism of Ganoderma lucidum Spores on Alleviation of Diabetic Cardiomyopathy in a Pilot in vivo Study. Diabetes Metab Syndr Obes. 2020;13:4809-4822. [13] ZHANG W, LEI Z, MENG J, et al. Water Extract of Sporoderm-Broken Spores of Ganoderma lucidum Induces Osteosarcoma Apoptosis and Restricts Autophagic Flux. Onco Targets Ther. 2019;12:11651-11665. [14] LI D, GAO L, LI M, et al. Polysaccharide from spore of Ganoderma lucidum ameliorates paclitaxel-induced intestinal barrier injury: Apoptosis inhibition by reversing microtubule polymerization. Biomed Pharmacother. 2020;130:110539. [15] JIAO C, CHEN W, TAN X, et al. Ganoderma lucidum spore oil induces apoptosis of breast cancer cells in vitro and in vivo by activating caspase-3 and caspase-9. J Ethnopharmacol. 2020;247:112256. [16] LIU Q, TIE L. Preventive and Therapeutic Effect of Ganoderma (Lingzhi) on Diabetes. Adv Exp Med Biol. 2019;1182:201-215. [17] CAO Y, XU X, LIU S, et al. Ganoderma: A Cancer Immunotherapy Review. Front Pharmacol. 2018;9:1217. [18] CAI M, LIANG X, LIU Y, et al. Transcriptional Dynamics of Genes Purportedly Involved in the Control of Meiosis, Carbohydrate, and Secondary Metabolism during Sporulation in Ganoderma lucidum. Genes (Basel). 2021;12(4):504. [19] HE Z, YIN G, LI QQ, et al. Diabetes Mellitus Causes Male Reproductive Dysfunction: A Review of the Evidence and Mechanisms. In Vivo. 2021; 35(5):2503-2511. [20] WU C, LI Y, ZHONG S, et al. ROS is essential for initiation of energy deprivation-induced autophagy. J Genet Genomics. 2021;48(6):512-515. [21] SOLGI T, AMIRI I, ASL SS, et al. Antiapoptotic and antioxidative effects of cerium oxide nanoparticles on the testicular tissues of streptozotocin-induced diabetic rats: An experimental study. Int J Reprod Biomed. 2021;19(7):589-598. [22] WIBLE DJ, BRATTON SB. Reciprocity in ROS and autophagic signaling. Curr Opin Toxicol. 2018;7:28-36. [23] YUAN Y, CHEN Y, PENG T, et al. Mitochondrial ROS-induced lysosomal dysfunction impairs autophagic flux and contributes to M1 macrophage polarization in a diabetic condition. Clin Sci (Lond). 2019;133(15):1759-1777. [24] TIAN Y, SONG W, XU D, et al. Autophagy Induced by ROS Aggravates Testis Oxidative Damage in Diabetes via Breaking the Feedforward Loop Linking p62 and Nrf2. Oxid Med Cell Longev. 2020;2020:7156579. [25] MAO H, CHEN W, CHEN L, et al. Potential role of mitochondria-associated endoplasmic reticulum membrane proteins in diseases. Biochem Pharmacol. 2022;199:115011. [26] GUO T, LIU T, SUN Y, et al. Sonodynamic therapy inhibits palmitate-induced beta cell dysfunction via PINK1/Parkin-dependent mitophagy. Cell Death Dis. 2019;10(6):457. [27] HUANG C, BIAN J, CAO Q, et al. The Mitochondrial Kinase PINK1 in Diabetic Kidney Disease. Int J Mol Sci. 2021;22(4):1525. [28] XIAO B, GOH JY, XIAO L, et al. Reactive oxygen species trigger Parkin/PINK1 pathway-dependent mitophagy by inducing mitochondrial recruitment of Parkin. J Biol Chem. 2017;292(40):16697-16708. [29] ZHOU P, XIE W, MENG X, et al. Notoginsenoside R1 Ameliorates Diabetic Retinopathy through PINK1-Dependent Activation of Mitophagy. Cells. 2019;8(3):213. [30] LI W, DU M, WANG Q, et al. FoxO1 Promotes Mitophagy in the Podocytes of Diabetic Male Mice via the PINK1/Parkin Pathway. Endocrinology. 2017;158(7):2155-2167. [31] GLADKOVA C, MASLEN SL, SKEHEL JM, et al. Mechanism of parkin activation by PINK1. Nature. 2018;559(7714):410-414. [32] WANG B, SHI Y, CHEN J, et al. High glucose suppresses autophagy through the AMPK pathway while it induces autophagy via oxidative stress in chondrocytes. Cell Death Dis. 2021;12(6):506. [33] YOU S, ZHENG J, CHEN Y, et al. Research progress on the mechanism of beta-cell apoptosis in type 2 diabetes mellitus. Front Endocrinol (Lausanne). 2022;13:976465. [34] XI J, RONG Y, ZHAO Z, et al. Scutellarin ameliorates high glucose-induced vascular endothelial cells injury by activating PINK1/Parkin-mediated mitophagy. J Ethnopharmacol. 2021;271:113855. [35] GREEN DR. The Death Receptor Pathway of Apoptosis. Cold Spring Harb Perspect Biol. 2022;14(2):a041053. [36] HAM J, LIM W, SONG G. Pendimethalin induces apoptosis in testicular cells via hampering ER-mitochondrial function and autophagy. Environ Pollut. 2021;278:116835. |
| [1] | Wang Liping, Lian Tianxing, Hu Yongrong, Yang Hongsheng, Zeng Zhimou, Liu Hao, Qu Bo. HU value of chest CT vertebral body in the opportunistic screening of type 2 diabetes mellitus osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(6): 950-954. |
| [2] | Yan Binghan, Li Zhichao, Su Hui, Xue Haipeng, Xu Zhanwang, Tan Guoqing. Mechanisms of traditional Chinese medicine monomers in the treatment of osteoarthritis by targeting autophagy [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 627-632. |
| [3] | Wang Jingfeng, Wen Dengtai, Wang Shijie, Gao Yinghui. Atg-mediated autophagy, exercise and skeletal muscle aging [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(2): 295-301. |
| [4] | Ma Suilu, He Zhijun, Liu Tao, Li Yan, He Yuanxu, He Bo, Wang Weiwei, Wei Xiaotao. Traditional Chinese medicine monomer in the prevention and treatment of flap necrosis by regulating “autophagy” [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(1): 153-158. |
| [5] | Deng Rui, Huang Keming, Luo Jian, Chen Gong, Feng Jian, Huang Weiyi, Wei Gang. Effect of heme oxygenase-1-mediated atorvastatin on macrophage polarization and cholesterol accumulation [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(1): 62-67. |
| [6] | Sun Jiajia, Zhu Haidi, Lu Yun, Zhang Kai. Comparison of bone metabolism markers between type 2 diabetes mellitus and non-type 2 diabetes mellitus patients with hip fracture [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1156-1160. |
| [7] | Wang Ji, Zhang Min, Yang Zhongya, Zhang Long. A review of physical activity intervention in type 2 diabetes mellitus with sarcopenia [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1272-1277. |
| [8] | Nie Chenchen, Su Kaiqi, Gao Jing, Fan Yongfu, Ruan Xiaodi, Yuan Jie, Duan Zhaoyuan, Feng Xiaodong. The regulatory role of circular RNAs in cerebral ischemia-reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1286-1291. |
| [9] | Li Long, Li Guangdi, Shi Hao, Deng Keqi. Circular RNA as a competing endogenous RNA is involved in the regulation of osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(5): 751-757. |
| [10] | Li Zhichao, Tan Guoqing, Su Hui, Xu Zhanwang, Xue Haipeng. Regulatory role of non-coding RNAs as potential therapeutic targets in spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(5): 758-764. |
| [11] | Wu Yujie, Wan Xiaofang, Wei Mianxing, Peng Shiyuan, Xu Xiaomei. Correlation between autophagy and the Hippo-YAP protein pathway in periodental ligament cells on the pressure side of a mouse model of orthodontic tooth movement [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(5): 683-689. |
| [12] | Yang Heng, Zheng Liying, Liu Zhili, Wang Qinzhang, Hao Zhiqiang, Wang Jingshen, Wang Yuan, Li Yongle, Tan Minghui, Zou Xiaofeng, Zhang Guoxi, Huang Ruohui, Jiang Bo, Qian Biao. Physiological function of the testis in rats undergoing different parts of vasectomy [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(5): 720-725. |
| [13] | Jin Hongguang, Shi Zhuang, Wang Huaxin, Ni Ma, Fu Yong. Mechanism of Mongolian Medicine Erden-uril on avascular necrosis of the femoral head in rats [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(35): 5670-5675. |
| [14] | Gu Yingchu, Gu Ye, Wu Zerui, Fang Tao, Wang Qiufei, Chen Bingqian, Peng Yuqin, Geng Dechun, Xu Yaozeng. Signaling pathway of osteoblast autophagy in periprosthetic osteolysis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(34): 5561-5569. |
| [15] | Zhang Qi, Li Guilin, He Yanjiao. Ultrastructural features and clinical significance of autophagosome in benign meningiomas [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(33): 5304-5308. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||