Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (7): 1076-1082.doi: 10.12307/2024.112
Previous Articles Next Articles
Extracellular vesicles carrying non-coding RNA regulate the activation of osteoclasts
Liu Jianhong1, Liao Shijie1, Li Boxiang2, Tang Shengping1, Wei Zhendi1, Ding Xiaofei1
- 1Division of Hand Surgery, Department of Trauma and Orthopedics, First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China; 2Department of Orthopedic Surgery, Affiliated Ethnic Hospital of Guangxi Medical University, Nanning 530001, Guangxi Zhuang Autonomous Region, China
-
Received:2023-02-10Accepted:2023-03-24Online:2024-03-08Published:2023-07-17 -
Contact:Liu Jianhong, Master candidate, Division of Hand Surgery, Department of Trauma and Orthopedics, First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China -
About author:Ding Xiaofei, MD, Chief physician, Division of Hand Surgery, Department of Trauma and Orthopedics, First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China
CLC Number:
Cite this article
Liu Jianhong, Liao Shijie, Li Boxiang, Tang Shengping, Wei Zhendi, Ding Xiaofei. Extracellular vesicles carrying non-coding RNA regulate the activation of osteoclasts[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1076-1082.
share this article
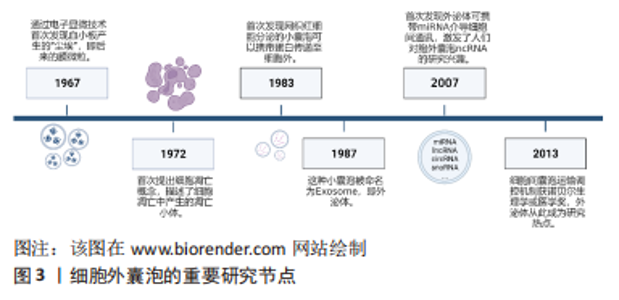
2.1 细胞外囊泡及非编码RNA概述 细胞外囊泡由多种类型的细胞释放,并且能够在血浆、全血、脐血、脑脊液、尿液、乳汁和唾液等体液中循环[10],按照直径大小的不同主要分为3类:外泌体、膜微粒(又称为微囊泡或外小体)及凋亡小体。外泌体由多囊泡体向内出芽产生,直径30-120 nm,表面标志物为CD63、CD83、Alix;膜微粒由细胞质膜向外出芽产生,直径100-1 000 nm,表面标志物为整合素、基质金属蛋白酶、CD40;凋亡小体则在细胞凋亡后期通过细胞质膜向外出芽产生,直径800-5 000 nm,表面标志物为Caspase3、组蛋白[8]。除以上提到的表面标志物以外,细胞外囊泡还携带了与其来源细胞相同的抗原,同时携带了来源细胞中的蛋白质、脂质、细胞器、编码RNA、非编码RNA、DNA等,因此能够反映来源细胞的生理状态。细胞外囊泡进入体液循环后,到达靶细胞,通过被靶细胞内吞、其携带的抗原与靶细胞膜上的受体结合、直接与靶细胞膜融合等3种方式,将其携带的物质释放,发挥细胞间通讯的作用,介导细胞之间的交流及病理生理活动[10]。图3为细胞外囊泡的重要研究节点。"
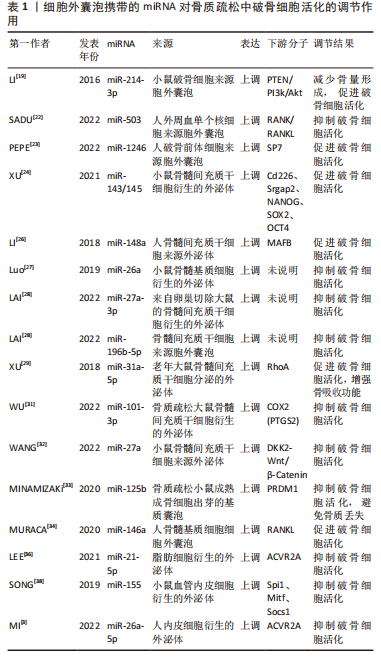
非编码RNA目前按其功能分为两类:一是参与核糖体构成的组成性非编码RNA,包括转运RNA(transfer RNA,tRNA)、核糖体RNA(ribosomal RNA,rRNA)、核小RNA(small nuclear RNA,snRNA)、核仁小RNA (small nucleolar RNA,snoRNA)、胞质小RNA (small cytoplasmic RNA, scRNA)、催化小RNA (ribozyme)等;另外一类是参与基因表达及转录后修饰的调控性非编码RNA,按其长度及形状又可分为20-200核苷酸的非编码小RNA(small non-coding RNA,sncRNA)如微小RNA(microRNA,miRNA)、piwi 相互作用RNA (piwi interacting RNA,piRNA)、小干扰RNA(Small interfering RNA,siRNA)等,还有超过200核苷酸的长链非编码RNA (long non-oding RNA,lncRNA)和环状RNA(circular RNA,circRNA) 等,它们不参与蛋白质的编码,是基因表达调控及转录后调控中的重要分子,对于维持细胞正常的生理功能有重要意义。 通过对相关文献的阅读发现,目前关于细胞外囊泡携带非编码RNA调控破骨细胞活化的研究多数集中于miRNA,其次为lncRNA及circRNA,其余非编码RNA罕见报道。miRNA的长度为18-25个核苷酸,研究表明,约有60%的人类蛋白质编码基因可能由miRNA调控[11],它们与靶基因的3’-UTR结合,导致mRNA降解和转录抑制。miRNA在体内调节大多数生物过程,包括细胞发育、分化、增殖、代谢以及细胞周期调节等,其中就包括破骨细胞活化相关基因的表达[12]。 lncRNA主要存在于细胞核或细胞质中,为众多重要生理现象和病理过程的关键调控因子[13]。lncRNA的功能被归结为分子机制的4种原型——信号功能、诱饵功能、引导功能、支架功能。在转录过程中,lncRNA可作为辅助因子调节转录因子活性;lncRNA还可以与特定蛋白质结合,调节蛋白质活性或改变其细胞质定位,甚至形成细胞亚结构或蛋白质复合物[14];同时 lncRNA还可参与其靶mRNA的调控和加工,如剪切、剪接或降解[15];此外,lncRNA可以作为“miRNA海绵”,与miRNA竞争性结合,从而保护它们的目标RNA不被降解或翻译抑制[16]。 circRNA是一种具有共价闭环结构的新型RNA,长度为数百至数千个核苷酸,与线性RNA相比,circRNA缺少5’帽与3’多聚腺苷尾,能够在一定程度上抵抗核酸外切酶的降解,因此其结构更稳定[17]。circRNA具有高度保守性,其功能大概可分为4种:“miRNA海绵”、与蛋白质相互作用、调节转录、参与翻译。circRNA参与多种生物活动的调控,近年来,越来越多的表观遗传学数据揭示了circRNA参与骨代谢的调控[18]。 2.2 细胞外囊泡携带的miRNA调控破骨细胞活化 miRNA一直以来都是非编码RNA中的研究热点,在细胞外囊泡携带非编码RNA调控破骨细胞活化的研究中,miRNA发挥的调控作用也已有大量研究报道,其中被研究最多的疾病为骨质疏松,其次为肿瘤的骨转移。研究表明,不同骨骼系统疾病中不同细胞来源的细胞外囊泡携带的miRNA表达水平基本异常上调,通过靶向磷酸酶和紧张素同源物(Phosphatase and tensin homologue,PTEN)/磷脂酰肌醇3-激酶(phosphatidylinositol 3 kinase,PI3K)/Akt、RANK/RANKL、NF-κB、NFATc1等通路调控破骨细胞活化。细胞外囊泡携带的miRNA对于调控破骨细胞活化的研究已达到相对成熟的阶段,在此基础上对miRNA更深层次的调控机制进行探索,将有助于推动各种骨骼系统疾病诊疗的发展步伐。 2.2.1 骨质疏松 在骨质疏松症中,破骨细胞来源的外泌体携带miR-214-3p,将其运输到成骨细胞,促进RANKL介导的破骨细胞分化[19],在小鼠体内抑制miR-214-3p的表达能促进骨量形成[20],miR-214-3p还能靶向抑制PTEN,激活PI3K/Akt通路,导致体内破骨细胞生成增强[21]。 绝经后骨质疏松患者外周血单个核细胞的细胞外囊泡miR-503可以抑制RANK介导的破骨细胞生成[22];同时,PEPE等[23]收集了骨质疏松、骨量减少患者及健康人的血清,从血清中提取细胞外囊泡后测序发现在表达差异的miRNA中,miR-1246在患者血清中显著上调,体外研究发现患者血清来源的细胞外囊泡能促进破骨细胞活化,提示外泌体miR-1246促进破骨活化,具体机制仍需深入探索。 骨髓间充质干细胞在调控破骨细胞活化中发挥了重要作用,其来源的细胞外囊泡携带miR-143/145促进破骨细胞形成[24],外泌体miR-148a可以抑制V-MAF肌肉腱膜纤维肉瘤癌基因同源物B(MAFB)的表达来促进破骨细胞形成[25-26];而miR-26a [27]、miR-27a-3p、miR-196b-5p抑制破骨形成[28]。XU的团队研究发现,老年大鼠骨髓间充质干细胞来源的外泌体中miR-31a-5p的表达水平明显高于年轻大鼠,并证实外泌体miR-31a-5p在体外可促进破骨细胞活化,增强骨吸收功能,可能促进了骨质疏松的发生发展[29-30]。经巴戟天多糖处理的骨质疏松大鼠模型的骨髓间充质干细胞来源外泌体中miR-101-3p表达升高,抑制了巨噬细胞向破骨细胞的分化和增殖[31]。小鼠骨髓间充质干细胞来源的细胞外囊泡中富含miR-27a,可以通过与DKK2结合促进骨形成并抑制巨噬细胞分化为破骨细胞,改善骨质疏松小鼠模型的骨丢失[32]。 骨质疏松小鼠模型中,从成熟成骨细胞出芽的基质囊泡(microvesicles,MVs)积聚在未矿化的骨基质(类骨)中,基质囊泡中的miR-125b能通过靶向PR结构域蛋白1(Prdm1)抑制破骨形成,避免骨质流失[33]。人骨髓基质细胞释放的细胞外囊泡也富集miRNA,敲除miR-146a的小鼠在经卵巢切除术诱导骨质疏松症后,骨组织参数与对照组小鼠无明显差异,说明抑制miR-146a的表达能够改善骨质疏松,进一步研究发现人骨髓基质细胞的细胞外囊泡miR-146a促进成骨细胞中的RANKL表达,从而促进破骨细胞活化[34-35]。 LEE等[36]发现脂肪组织分泌的细胞外囊泡可以通过其携带的miR-21-5p通过靶向激活素受体2A(ACVR2A),抑制RAW264.7细胞的破骨细胞分化,从而改善骨质疏松的症状。另有研究表明,血管内皮细胞来源的细胞外囊泡比成骨细胞或骨髓间充质干细胞来源的细胞外囊泡具有更有效的骨靶向性,并可通过miR-155抑制破骨细胞的活性和分化,提示含有miR-155的细胞外囊泡可能是对抗骨质疏松症的潜在靶点[10,37-38];MI等[3]的研究也证明了内皮细胞外泌体来源的miR-26a-5p能够抑制破骨活化表型。 作者将以上综述的细胞外囊泡携带miRNA在骨质疏松中调控破骨细胞活化的相关研究整理于表1。"
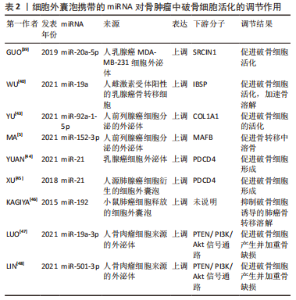
2.2.2 骨肿瘤 骨为多种肿瘤转移的好发部位,肿瘤细胞分泌的细胞外囊泡携带miRNA调控破骨细胞活化,影响骨转移溶解性病灶的进展。一项乳腺癌骨转移的研究表明,携带miR-20a-5p的人乳腺癌MDA-MB-231细胞外泌体干预骨髓巨噬细胞后,靶向SRCIN1,促进破骨细胞的活化[22,37,39]。在另一项雌激素受体阳性的乳腺癌骨转移细胞的研究中观察到外泌体miR-19a和一类分泌因子整合素结合唾液蛋白(IBSP)同步上调,外泌体miR-19a的表达水平增加与诱导骨溶解性骨转移有关——外泌体miR-19a被IBSP招募到肿瘤微环境中,促进破骨细胞活化,从而加速骨溶解病变的形成[40]。KAGIYA[41-42]的一项研究表明,在破骨细胞来源的细胞外囊泡中发现了包括let-7e、miR-21、miR-155、miR-210、miR-223和miR-378等几种miRNA,其中miR-378可促进细胞存活并且在促进破骨细胞活化过程中起重要作用,由破骨细胞活性过度引起的溶骨性骨转移常发生在乳腺癌晚期,乳腺癌骨转移患者的血清中也检测到miR-378高表达。 前列腺癌也为多发骨转移的一种肿瘤,形成溶骨性病灶,研究发现,癌细胞分泌的外泌体miR-92a-1-5p直接靶向COL1A1,下调Ⅰ型胶原蛋白的表达,促进破骨细胞的活化[43];外泌体miR-152-3p则通过靶向破骨细胞生成调节因子MAFB,促进骨转移中溶骨进展[5]。 miR-21在肺腺癌细胞衍生的细胞外囊泡以及乳腺癌细胞外泌体中表达升高,通过靶向程序性细胞死亡蛋白4(PDCD4)促进破骨细胞形成,同时临床数据显示,较高水平的外泌体miR-21与肺腺癌患者的总生存率较差有关[37,44-45]。另有研究表明,小鼠高转移性肺癌细胞过表达miR-192后,其释放的细胞外囊泡携带miR-192能够抑制破骨细胞诱导的肺癌骨转移溶解性病灶[46]。 在骨原位肿瘤如骨肉瘤中,肿瘤细胞来源的外泌体miR-501-3p、miR-19a-3p通过激活PTEN/ PI3K/Akt信号通路,促进破骨细胞产生并加重骨缺损[47-49]。 作者将以上综述的细胞外囊泡携带miRNA在骨肿瘤中调控破骨细胞活化的相关研究整理于表2。"
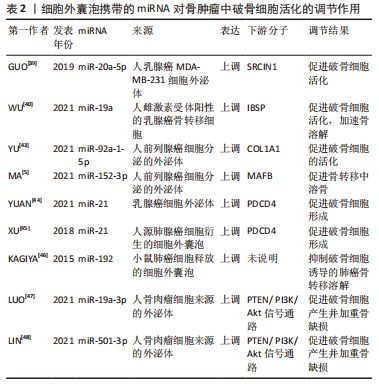

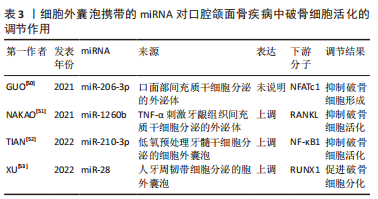
2.2.3 口腔颌面骨疾病 口面部间充质干细胞分泌的外泌体携带miR-206-3p,受一种在骨骼发育中发挥重要作用的锌指转录因子GATA结合蛋白4(GATA4)调控,靶向NFATc1,抑制破骨细胞形成,可能为改善颅面骨发育畸形的一个靶点[50]。肿瘤坏死因子α刺激牙龈组织间充质干细胞分泌的外泌体miR-1260b通过抑制RANKL信号通路抑制破骨细胞活化[51]。TIAN等[52]使用低氧预处理牙髓干细胞后发现其分泌的细胞外囊泡中含有miR-210-3p,能促进巨噬细胞向M2型分化,抑制炎症,同时miR-210-3p还通过靶向NF-κB通路抑制破骨细胞活化,可能作为其他炎症性骨溶解疾病一个治疗靶点。细胞外囊泡miRNA在口腔正畸中调控破骨细胞的作用也已被报道,人牙周韧带细胞分泌的细胞外囊泡携带miR-28促进破骨活化,进而抑制正畸治疗中牙齿的移动[54]。 作者将以上综述的细胞外囊泡携带miRNA在口腔颌面骨疾病中调控破骨细胞活化的相关研究整理于表3。"

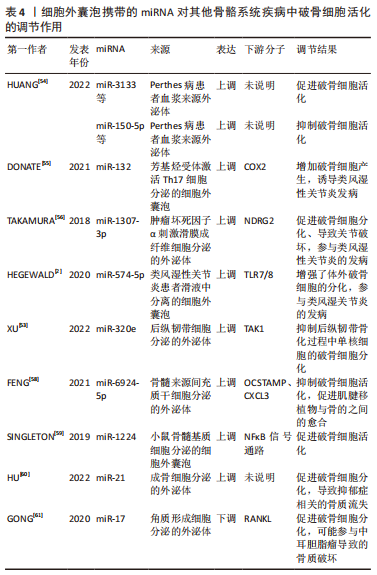
2.2.4 其他骨骼系统疾病 Perthes病是小儿骨科中最常见的髋关节疾病,其发病机制可能与破骨细胞过度激活有关,作者所在课题组收集了患儿及健康对照组儿童的血浆,随后提取血浆中的外泌体进行测序,发现miR-3133、miR-4644、miR-150-5p、miR-4693-3p、miR-4693-5p、miR-141-3p、miR-504-5p、miR-7154-5p、miR-4709-5p、miR-30a-3p等miRNA在患儿血浆外泌体中表达显著升高,进一步体外实验发现miR-3133、miR-4693-3p、miR-4693-5p、miR-141-3p、miR-30a-3p促进破骨细胞活化,而miR-150-5p、miR-7154-5p抑制破骨细胞活化[54],研究结果说明了以上miRNA可能作为该病的治疗靶点。 在骨关节炎中,由芳基烃受体激活Th17细胞分泌的细胞外囊泡中携带了miR-132,其作为促炎递质减少了COX2的产生,从而增加破骨细胞的产生[55]。TAKAMURA 等[56]发现,有4种miRNA(miR-155-5p,miR-146a-5p,miR-323a-5p、miR-1307-3p)在肿瘤坏死因子α刺激的滑膜成纤维细胞来源的外泌体中上调,其中miR-1307-3p可能通过靶向N-myc下游调节基因2(NDRG2)促进破骨细胞分化并导致关节破坏,参与类风湿性关节炎的发病。HEGEWALD团队[2,57]从类风湿性关节炎患者滑液中分离的细胞外囊泡显著增强了体外破骨细胞的分化,这种效应可以归因于细胞外囊泡递送的miR-574-5p激活跨膜受体TLR7/8的信号传导而显著增加破骨细胞形成。 在后纵韧带骨化中,XU等[53]发现后纵韧带分泌的外泌体miR-320e能直接靶向TAK1抑制破骨形成。手术修复前交叉韧带损伤时,肌腱移植物需要插入骨隧道内,此时常常观察到骨隧道周围的骨溶解,关于此术式造成骨溶解的一项研究证明了过表达Scleraxis基因的骨髓来源间充质干细胞能分泌外泌体携带miR-6924-5p,抑制破骨细胞活化,促进肌腱移植物与骨的之间的愈合[58]。 创伤性颅脑损伤患者并发骨量减少或骨折较为常见,SINGLETON等[59]的研究发现,在皮质受冲击的小鼠中,与破骨细胞活化相关的NFκB信号通路失调,体外实验证明来自皮质受损的小鼠骨髓基质细胞的细胞外囊泡中miR-1224显著上调,且能激活NFκB通路,促进破骨细胞活化,由此解释了创伤性颅脑损伤患者骨量丢失的可能机制。 交感神经肾上腺素能激活与应激诱导的抑郁症有关,并可导致骨丢失, 研究表明交感神经应激通过β1/2-肾上腺素能受体(β1/2-AR)信号传导触发成骨细胞中的miR-21通过外泌体转移到破骨细胞祖细胞,促进骨质减少并破坏骨稳态, 此线索有助于建立可行的策略来抵消心理压力下的骨丢失[60]。中耳胆脂瘤是一种囊性病变,来源于角化层状鳞状上皮,其中骨破坏是最重要的特征,GONG等[61]发现角质形成细胞衍生外泌体中miRNA-17表达下调,可以上调成纤维细胞RANKL的表达并促进破骨细胞分化。 作者将以上综述的细胞外囊泡携带miRNA在其他骨骼系统疾病中调控破骨细胞活化的相关研究整理于表4。"
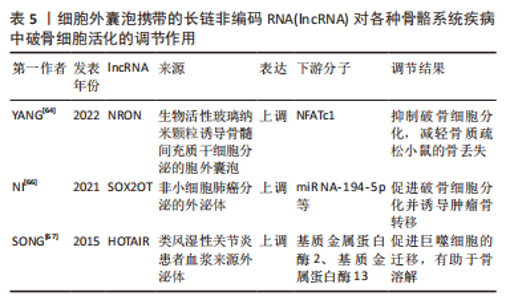
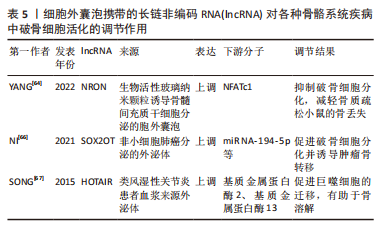
综上所述,细胞外囊泡携带的miRNA对于不同疾病中调控破骨细胞的活化发挥了举足轻重的作用,其作为相关疾病治疗靶点具有无限可能。但大部分的研究仍缺乏一定的深度,大多处于对破骨细胞活化表型的调控及下游分子的探索阶段,上游机制的探索还需通过进一步的研究阐明,以此提供更完整、更有说服力的理论依据。 2.3 细胞外囊泡携带的lncRNA调控破骨细胞活化 细胞外囊泡携带的非编码RNA在骨骼系统疾病中调控破骨细胞活化的相关研究也囊括了lncRNA,目前研究表明,lncRNA在调控破骨细胞活化的机制主要可分为2种,一是发挥ceRNA的作用,通过海绵吸附下游的miRNA,靶向破骨细胞活化相关的基因;二则结合转录因子等多种蛋白,以此调控破骨细胞活化。 lncRNA在骨质疏松症的疾病发展中发挥着重要的调节作用,例如lncRNA ZBTB40-IT1可通过调节骨代谢相关基因的表达而抑制骨形成并促进破骨细胞生成;lncRNA AK077216在破骨形成过程中显著上调等[34,62-63]。生物活性玻璃纳米颗粒(bioactive glass nanoparticles,BGN)由于其特殊的成骨活性而在骨组织修复中的应用日益受到关注,YANG等[64]发现,BGN可促进骨髓间充质干细胞分泌更多的细胞外囊泡(BGN+BMSC EVs),进而在体外抑制破骨细胞分化,机制上可能是BGN+BMSC EVs富含的lncRNA NRON通过与活化T细胞转录因子的核因子结合来抑制NFATc1的核转位,从而抑制破骨细胞分化,减轻骨质疏松小鼠的骨丢失。当肿瘤骨转移发展时,骨内肿瘤细胞会产生一系列溶骨因子,这些溶骨因子包括结缔组织生长因子(CTGF)等,它们触发源自单核细胞的破骨细胞前体的分化,研究发现 lncRNA也会参与这一过程[65]。NI等[66]的研究显示,非小细胞肺癌衍生的外泌体lncRNA-SOX2OT通过靶向破骨细胞中的miR-194-5p/RAC1信号轴和TGF-β/pTHrP/RANKL信号通路,从而促进破骨细胞分化并诱导肿瘤骨转移,这可能成为转移性非小细胞肺癌的预后生物标志物和治疗靶点。SONG等[67]探寻了在类风湿性关节炎中具有预后价值的lncRNA,他们发现患者的血浆外泌体lncRNA HOTAIR相比于对照组显著增高,并且能促进巨噬细胞的迁移能力,lncRNA HOTAIR能激活破骨细胞和类风湿性关节炎滑膜细胞中的基质金属蛋白酶2和基质金属蛋白酶13,这可能有助于骨与软骨基质的溶解,从而促进关节破坏而导致疾病发生。 综上,细胞外囊泡携带lncRNA调控破骨细胞活化相关的研究数量目前未及miRNA的相关研究数量,但可以从目前已有的研究中看到lncRNA在各种骨骼系统疾病中调控破骨细胞也起到了重要的作用,提示细胞外囊泡携带的lncRNA也可能作为疾病的诊断、治疗、预后的关键靶点,且对于其调控破骨细胞活化的机制仍需更多的研究去挖掘。 作者将以上综述的细胞外囊泡携带lncRNA在各类骨骼系统疾病中调控破骨细胞活化的相关研究整理于表5。"

2.4 细胞外囊泡携带的circRNA调控破骨细胞活化 最近研究发现参与调控破骨细胞活化生理过程的非编码RNA不只是lncRNA和miRNA,circRNA也可能通过外泌体调控破骨细胞功能参与介导骨稳态的平衡,在骨稳态过程发挥重要作用,其潜在机制主要涉及在分子水平上circRNA与非编码RNA分子的相互作用而在转录水平调节基因表达并调控破骨活化过程的信号通路。circRNA被发现在破骨细胞分化过程中上调表达,同时miRNA表达也上调,而lncRNA表达量下调,表明circRNA-miRNA-lncRNA构成的ceRNA互作网络在破骨分化中或起重要作用[68]。另有研究发现,小鼠MC3T3-E1细胞来源的外泌体中RNA去甲基化酶ALKBH5抑制circ_0008542与miRNA-185-5p的结合以负调控骨吸收过程[69]。circ_0008542可在骨结合微环境中通过外泌体作用于miR-185-5p/RANK轴调控成骨细胞和破骨细胞之间的通信网络,因而促进了口腔植入物周围的破骨细胞过度活化,造成植入物周围的骨吸收,暗示circRNA作为一种重要的致病分子参与疾病的发生和进展。虽然细胞外囊泡携带circRNA调控破骨细胞活化的相关研究较少,但根据已有文献可知,circRNA能作为ceRNA网络中的一个关键环节将lncRNA及miRNA联系起来,参与破骨细胞活化的调控,其作为疾病诊疗靶点的潜力也不容小觑。 2.5 细胞外囊泡携带的其他ncRNA调控破骨细胞活化 目前其他非编码RNA通过胞外囊泡介导破骨细胞活化的研究较少。已有研究表明 snoRNA在肿瘤骨转移及骨稳态维持中发挥作用,如snoRNA-SNORD32A的表达被发现可能与破骨细胞状态有关;snoRNA可能有助于p53诱导的骨肉瘤转移,提示snoRNA与破骨细胞/成骨细胞的平衡失调密切相关[70]。此外,有研究还发现人类外周血中的细胞外囊泡可携带包括SNORA74A在内的几种snoRNA,并可作为潜在的生物标志物[71]。但目前仍然缺乏胞外囊泡携带snoRNA调控破骨细胞活化的相关研究,这也是未来学者们可以深入探索的研究方向。"

| [1] HU Y, XU R, CHEN C, et al. Extracellular vesicles from human umbilical cord blood ameliorate bone loss in senile osteoporotic mice. Metabolism. 2019;95:93-101. [2] HEGEWALD AB, BREITWIESER K, OTTINGER SM, et al. Extracellular miR-574-5p Induces Osteoclast Differentiation via TLR 7/8 in Rheumatoid Arthritis. Front Immunol. 2020;11:585282. [3] MI B, CHEN L, XIONG Y, et al. Osteoblast/Osteoclast and Immune Cocktail Therapy of an Exosome/Drug Delivery Multifunctional Hydrogel Accelerates Fracture Repair. ACS Nano. 2022;16(1):771-782. [4] PAVONE V, CHISARI E, VESCIO A, et al. Aetiology of Legg-Calvé-Perthes disease: A systematic review. World J Orthop. 2019;10(3):145-165. [5] MA Q, LIANG M, WU Y, et al. Small extracellular vesicles deliver osteolytic effectors and mediate cancer-induced osteolysis in bone metastatic niche. J Extracell Vesicles. 2021;10(4):e12068. [6] SOBACCHI C, SCHULZ A, COXON FP, et al. Osteopetrosis: genetics, treatment and new insights into osteoclast function. Nat Rev Endocrinol. 2013;9(9): 522-536. [7] LU B, JIAO Y, WANG Y, et al. A FKBP5 mutation is associated with Paget’s disease of bone and enhances osteoclastogenesis. Exp Mol Med. 2017; 49(5):e336. [8] LIU M, SUN Y, ZHANG Q. Emerging Role of Extracellular Vesicles in Bone Remodeling. J Dent Res. 2018;97(8):859-868. [9] 陈逸青,章秋,代芳.外泌体在骨重建以及骨质疏松中的研究进展[J].中国骨质疏松杂志,2020,26(1):129-134. [10] LI Q, LI C, ZHANG W, et al. Potential Effects of Exosomes and their MicroRNA Carrier on Osteoporosis. 2022;28(11):899-909. [11] FENG Q, ZHENG S, ZHENG J. The emerging role of microRNAs in bone remodeling and its therapeutic implications for osteoporosis. Biosci Rep. 2018; 38(3):BSR20180453. [12] KO NY, CHEN LR, CHEN KH. The Role of Micro RNA and Long-Non-Coding RNA in Osteoporosis. Int J Mol Sci. 2020;21(14):4886. [13] PONTING CP, OLIVER PL, REIK W. Evolution and functions of long noncoding RNAs. Cell. 2009;136(4):629-641. [14] GOODALL GJ, WICKRAMASINGHE VO. RNA in cancer. Nat Rev Cancer. 2021; 21(1): 22-36. [15] SHI X, SUN M, LIU H, et al. Long non-coding RNAs: a new frontier in the study of human diseases. Cancer Lett. 2013;339(2):159-166. [16] WILUSZ JE, SUNWOO H, SPECTOR DL. Long noncoding RNAs: functional surprises from the RNA world. Genes Dev. 2009;23(13):1494-1504. [17] GAO M, ZHANG Z, SUN J, et al. The roles of circRNA-miRNA-mRNA networks in the development and treatment of osteoporosis. Front Endocrinol (Lausanne). 2022;13:945310. [18] OTON-GONZALEZ L, MAZZIOTTA C, IAQUINTA MR, et al. Genetics and Epigenetics of Bone Remodeling and Metabolic Bone Diseases. Int J Mol Sci. 2022;23(3):1500. [19] LI D, LIU J, GUO B, et al. Osteoclast-derived exosomal miR-214-3p inhibits osteoblastic bone formation. Nat Commun. 2016;7:10872. [20] HOLLIDAY LS, MCHUGH KP, ZUO J, et al. Exosomes: novel regulators of bone remodelling and potential therapeutic agents for orthodontics. Orthod Craniofac Res. 2017;20 Suppl 1(Suppl 1):95-99. [21] HUANG X, XIONG X, LIU J, et al. MicroRNAs-containing extracellular vesicles in bone remodeling: An emerging frontier. Life Sci. 2020;254:117809. [22] SADU L, KRISHNAN RH, AKSHAYA RL, et al. Exosomes in bone remodeling and breast cancer bone metastasis. Prog Biophys Mol Biol. 2022;175:120-130. [23] PEPE J, ROSSI M, BATTAFARANO G, et al. Characterization of Extracellular Vesicles in Osteoporotic Patients Compared to Osteopenic and Healthy Controls. J Bone Miner Res. 2022;37(11):2186-2200. [24] XU R, SHEN X, XIE H, et al. Identification of the canonical and noncanonical role of miR-143/145 in estrogen-deficient bone loss. Theranostics. 2021; 11(11): 5491-510. [25] LI Y, JIN D, XIE W, et al. Mesenchymal Stem Cells-Derived Exosomes: A Possible Therapeutic Strategy for Osteoporosis. Curr Stem Cell Res Ther. 2018;13(5):362-368. [26] XIE Y, CHEN Y, ZHANG L, et al. The roles of bone-derived exosomes and exosomal microRNAs in regulating bone remodelling. J Cell Mol Med. 2017; 21(5):1033-1041. [27] LUO ZW, LI FX, LIU YW, et al. Aptamer-functionalized exosomes from bone marrow stromal cells target bone to promote bone regeneration. Nanoscale. 2019;11(43):20884-20892. [28] LAI G, ZHAO R, ZHUANG W, et al. BMSC-derived exosomal miR-27a-3p and miR-196b-5p regulate bone remodeling in ovariectomized rats. PeerJ. 2022;10:e13744. [29] XU R, SHEN X, SI Y, et al. MicroRNA-31a-5p from aging BMSCs links bone formation and resorption in the aged bone marrow microenvironment. Aging Cell. 2018;17(4):e12794. [30] FOESSL I, KOTZBECK P, OBERMAYER-PIETSCH B. miRNAs as novel biomarkers for bone related diseases. J Lab Precis Med. 2019;4:2-13. [31] WU P, JIAO F, HUANG H, et al. Morinda officinalis polysaccharide enable suppression of osteoclastic differentiation by exosomes derived from rat mesenchymal stem cells. Pharm Biol. 2022;60(1):1303-1316. [32] WANG Y, ZHOU X, WANG D. Mesenchymal Stem Cell-Derived Extracellular Vesicles Inhibit Osteoporosis via MicroRNA-27a-Induced Inhibition of DKK2-Mediated Wnt/β-Catenin Pathway. Inflammation. 2022;45(2):780-799. [33] MINAMIZAKI T, NAKAO Y, IRIE Y, et al. The matrix vesicle cargo miR-125b accumulates in the bone matrix, inhibiting bone resorption in mice. Commun Biol. 2020;3(1):30. [34] MURACA M, CAPPARIELLO A. The Role of Extracellular Vesicles (EVs) in the Epigenetic Regulation of Bone Metabolism and Osteoporosis. Int J Mol Sci. 2020;21(22):8682. [35] ZHAO J, HUANG M, ZHANG X, et al. MiR-146a Deletion Protects From Bone Loss in OVX Mice by Suppressing RANKL/OPG and M-CSF in Bone Microenvironment. J Bone Miner Res. 2019;34(11):2149-2161. [36] LEE KS, LEE J, KIM HK, et al. Extracellular vesicles from adipose tissue-derived stem cells alleviate osteoporosis through osteoprotegerin and miR-21-5p. J Extracell Vesicles. 2021;10(12):e12152. [37] HE Y, WUERTZ-KOZAK K, KUEHL L, et al. Extracellular Vesicles: Potential Mediators of Psychosocial Stress Contribution to Osteoporosis? Int J Mol Sci. 2021;22(11):5846. [38] SONG H, LI X, ZHAO Z, et al. Reversal of Osteoporotic Activity by Endothelial Cell-Secreted Bone Targeting and Biocompatible Exosomes. Nano Lett. 2019;19(5):3040-3048. [39] GUO L, ZHU Y, LI L, et al. Breast cancer cell-derived exosomal miR-20a-5p promotes the proliferation and differentiation of osteoclasts by targeting SRCIN1. Cancer Med. 2019;8(12):5687-5701. [40] WU K, FENG J, LYU F, et al. Exosomal miR-19a and IBSP cooperate to induce osteolytic bone metastasis of estrogen receptor-positive breast cancer. Nat Commun. 2021;12(1):5196. [41] LI Q, HUANG Q, WANG Y, et al. Extracellular vesicle-mediated bone metabolism in the bone microenvironment. J Bone Miner Metab. 2018;36(1):1-11. [42] KAGIYA T. MicroRNAs: Potential Biomarkers and Therapeutic Targets for Alveolar Bone Loss in Periodontal Disease. Int J Mol Sci. 2016;17(8):1317. [43] YU L, SUI B, FAN W, et al. Exosomes derived from osteogenic tumor activate osteoclast differentiation and concurrently inhibit osteogenesis by transferring COL1A1-targeting miRNA-92a-1-5p. J Extracell Vesicles. 2021;10(3):e12056. [44] YUAN X, QIAN N, LING S, et al. Breast cancer exosomes contribute to pre-metastatic niche formation and promote bone metastasis of tumor cells. Theranostics. 2021;11(3):1429-1445. [45] XU Z, LIU X, WANG H, et al. Lung adenocarcinoma cell-derived exosomal miR-21 facilitates osteoclastogenesis. Gene. 2018;666:116-122. [46] KAGIYA T. MicroRNAs and Osteolytic Bone Metastasis: The Roles of MicroRNAs in Tumor-Induced Osteoclast Differentiation. J Clin Med. 2015; 4(9):1741-1752. [47] LUO T, ZHOU X, JIANG E, et al. Osteosarcoma Cell-Derived Small Extracellular Vesicles Enhance Osteoclastogenesis and Bone Resorption Through Transferring MicroRNA-19a-3p. Front Oncol. 2021;11:618662. [48] LIN L, WANG H, GUO W, et al. Osteosarcoma-derived exosomal miR-501-3p promotes osteoclastogenesis and aggravates bone loss. Cell Signal. 2021; 82:109935. [49] LI S. The basic characteristics of extracellular vesicles and their potential application in bone sarcomas. J Nanobiotechnology. 2021;19(1):277. [50] GUO S, GU J, MA J, et al. GATA4-driven miR-206-3p signatures control orofacial bone development by regulating osteogenic and osteoclastic activity. Theranostics. 2021;11(17):8379-8395. [51] NAKAO Y, FUKUDA T, ZHANG Q, et al. Exosomes from TNF-α-treated human gingiva-derived MSCs enhance M2 macrophage polarization and inhibit periodontal bone loss. Acta Biomater. 2021;122:306-324. [52] TIAN J, CHEN W, XIONG Y, et al. Small extracellular vesicles derived from hypoxic preconditioned dental pulp stem cells ameliorate inflammatory osteolysis by modulating macrophage polarization and osteoclastogenesis. Bioact Mater. 2022;22:326-342. [53] XU C, ZHANG Z, LIU N, et al. Small extracellular vesicle-mediated miR-320e transmission promotes osteogenesis in OPLL by targeting TAK1. Nat Commun. 2022;13(1):2467. [54] HUANG Q, LI B, LIN C, et al. MicroRNA sequence analysis of plasma exosomes in early Legg-Calvé-Perthes disease. Cell Signal. 2022;91:110184. [55] DONATE PB, ALVES DE LIMA K, PERES RS, et al. Cigarette smoke induces miR-132 in Th17 cells that enhance osteoclastogenesis in inflammatory arthritis. Proc Natl Acad Sci U S A. 2021;118(1):e2017120118. [56] TAKAMURA Y, AOKI W, SATOMURA A, et al. Small RNAs detected in exosomes derived from the MH7A synovial fibroblast cell line with TNF-α stimulation. PloS one. 2018;13(8):e0201851. [57] CHANG C, XU L, ZHANG R, et al. MicroRNA-Mediated Epigenetic Regulation of Rheumatoid Arthritis Susceptibility and Pathogenesis. Front Immunol. 2022;13:838884. [58] FENG W, JIN Q, MING-YU Y, et al. MiR-6924-5p-rich exosomes derived from genetically modified Scleraxis-overexpressing PDGFRα(+) BMMSCs as novel nanotherapeutics for treating osteolysis during tendon-bone healing and improving healing strength. Biomaterials. 2021;279:121242. [59] SINGLETON Q, VAIBHAV K, BRAUN M, et al. Bone Marrow Derived Extracellular Vesicles Activate Osteoclast Differentiation in Traumatic Brain Injury Induced Bone Loss. Cells. 2019;8(1):63. [60] HU CH, SUI B D, LIU J, et al. Sympathetic Neurostress Drives Osteoblastic Exosomal MiR-21 Transfer to Disrupt Bone Homeostasis and Promote Osteopenia. Small Methods. 2022;6(3):e2100763. [61] GONG N, ZHU W, XU R, et al. Keratinocytes-derived exosomal miRNA regulates osteoclast differentiation in middle ear cholesteatoma. Biochem Biophys Res Commun. 2020;525(2):341-347. [62] MEI B, WANG Y, YE W, et al. LncRNA ZBTB40-IT1 modulated by osteoporosis GWAS risk SNPs suppresses osteogenesis. Hum Genet. 2019;138(2):151-166. [63] LIU C, CAO Z, BAI Y, et al. LncRNA AK077216 promotes RANKL-induced osteoclastogenesis and bone resorption via NFATc1 by inhibition of NIP45. J Cell Physiol. 2019;234(2):1606-1617. [64] YANG Z, LIU X, ZHAO F, et al. Bioactive glass nanoparticles inhibit osteoclast differentiation and osteoporotic bone loss by activating lncRNA NRON expression in the extracellular vesicles derived from bone marrow mesenchymal stem cells. Biomaterials. 2022;283:121438. [65] LI C, WANG S, XING Z, et al. A ROR1-HER3-lncRNA signalling axis modulates the Hippo-YAP pathway to regulate bone metastasis. Nat Cell Biol. 2017; 19(2):106-119. [66] NI J, ZHANG X, LI J, et al. Tumour-derived exosomal lncRNA-SOX2OT promotes bone metastasis of non-small cell lung cancer by targeting the miRNA-194-5p/RAC1 signalling axis in osteoclasts. Cell Death Dis. 2021;12(7):662. [67] SONG J, KIM D, HAN J, et al. PBMC and exosome-derived Hotair is a critical regulator and potent marker for rheumatoid arthritis. Clin Exp Med. 2015; 15(1):121-126. [68] DOU C, CAO Z, YANG B, et al. Changing expression profiles of lncRNAs, mRNAs, circRNAs and miRNAs during osteoclastogenesis. Sci Rep. 2016;6: 21499. [69] WANG W, QIAO S, WU X, et al. Circ_0008542 in osteoblast exosomes promotes osteoclast-induced bone resorption through m6A methylation. Cell Death Dis. 2021;12(7):628. [70] PUPPO M, JAAFAR M, DIAZ JJ, et al. MiRNAs and snoRNAs in Bone Metastasis: Functional Roles and Clinical Potential. Cancers (Basel). 2022;15(1):242. [71] RAI AK, RAJAN KS, BISSERIER M, et al. Spaceflight-Associated Changes of snoRNAs in Peripheral Blood Mononuclear Cells and Plasma Exosomes-A Pilot Study. Front Cardiovasc Med. 2022;9:886689. |
| [1] | Liu Hanfeng, Wang Jingjing, Yu Yunsheng. Artificial exosomes in treatment of myocardial infarction: current status and prospects [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1118-1123. |
| [2] | Liu Tao, He Zhijun, Li Jinpeng, Song Yuan, Yao Xingzhang, Chen Wen, Li Yan, Bai Bihui. Role and mechanism of noncoding RNA in diabetic peripheral neuropathy [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1124-1129. |
| [3] | Zhuge Xiaoxuan, Li Ce, Bao Guangjie, Kang Hong. Potential value of canonical and non-canonical roles of connexin 43 in disease treatment [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1130-1136. |
| [4] | Feng Ruiqin, Han Na, Zhang Meng, Gu Xinyi, Zhang Fengshi. Combination of 1% platelet-rich plasma and bone marrow mesenchymal stem cells improves the recovery of peripheral nerve injury [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 985-992. |
| [5] | Wei Yuanxun, Chen Feng, Lin Zonghan, Zhang Chi, Pan Chengzhen, Wei Zongbo. The mechanism of Notch signaling pathway in osteoporosis and its prevention and treatment with traditional Chinese medicine [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 587-593. |
| [6] | He Yuanjie, Chen Yuheng, Zhao Yongchao, Wang Zhenglong. Progress in epigenetic regulation of vascular smooth muscle cell remodeling in the occurrence and development of aortic aneurysms [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 602-608. |
| [7] | Zhou Shuliang, Xu Liang, Qian Xuefeng, Zeng Jincai, Zhu Lifan. Correlation between the expression of miRNA-142-3p, mixed lineage kinase 3 and interleukin-1beta in nucleus pulposus and the degree of lumbar intervertebral disc degeneration [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(2): 165-171. |
| [8] | Guo Xiangying, Peng Zifu, He Yimin, Fang Hongbo, Jiang Ning. MiRNA-122 contributes to the effect of exercise on non-alcoholic fatty liver [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(2): 272-279. |
| [9] | Long Yi, Yang Jiaming, Ye Hua, Zhong Yanbiao, Wang Maoyuan. Extracellular vesicles in sarcopenic obesity: roles and mechanisms [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(2): 315-320. |
| [10] | Wang Xianfeng, Wang Kun, Sun Han, Sun Xiaoliang, Yan Litao. Mechanism underlying exosomal lncRNA H19 derived from umbilical cord mesenchymal stem cells promotes cartilage injury repair [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(1): 20-25. |
| [11] | Zhou Minghua, Hu Xiaoyu. LncRNA SNHG4 regulates miR-152-3p during osteoblastic differentiation of periodontal ligament stem cells [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(1): 38-43. |
| [12] | Guo Shuhui, Yang Ye, Jiang Yangyang, Xu Jianwen. Screening and validation of neurogenic bladder miRNA-mRNA regulatory network [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(在线): 1-8. |
| [13] | Pan Zhongjie, Qin Zhihong, Zheng Tiejun, Ding Xiaofei, Liao Shijie. Targeting of non-coding RNAs in the pathogenesis of the osteonecrosis of the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1441-1447. |
| [14] | Dang Yi, Du Chengyan, Yao Honglin, Yuan Nenghua, Cao Jin, Xiong Shan, Zhang Dingmei, Wang Xin. Hormonal osteonecrosis and oxidative stress [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1469-1476. |
| [15] | Long Guiyue, Li Dongdong, Liao Hongbing. Calcium phosphate cement/poly(lactic-co-glycolic acid) degradation products promote osteoclast differentiation of mouse monocytes [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1193-1198. |
| Viewed | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
Full text 185
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
Abstract 352
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||