Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (25): 4079-4086.doi: 10.12307/2024.193
Previous Articles Next Articles
Current status and future of treatment of pulmonary fibrosis by mesenchymal stem cells and extracellular vesicles
Wang Yanyang1, 2, Liu Chan1, 2, Yu Limei1, 2, He Zhixu1, 2, 3
- 1Key Cell Engineering Laboratory, Affiliated Hospital of Zunyi Medical University, Zunyi 563000, Guizhou Province, China; 2Ministry of Tissue Damage Repair and Regenerative Medicine Jointly Established a Collaborative Innovation Center, Zunyi Medical University, Zunyi 563000, Guizhou Province, China; 3Guizhou Children’s Hospital; Department of Pediatrics, Affiliated Hospital of Zunyi Medical University, Zunyi 563000, Guizhou Province, China
-
Received:2023-07-08Accepted:2023-08-24Online:2024-09-08Published:2023-11-24 -
Contact:He Zhixu, Chief physician, Professor, Doctoral supervisor, Key Cell Engineering Laboratory, Affiliated Hospital of Zunyi Medical University, Zunyi 563000, Guizhou Province, China; Ministry of Tissue Damage Repair and Regenerative Medicine Jointly Established a Collaborative Innovation Center, Zunyi Medical University, Zunyi 563000, Guizhou Province, China; Guizhou Children’s Hospital; Department of Pediatrics, Affiliated Hospital of Zunyi Medical University, Zunyi 563000, Guizhou Province, China -
About author:Wang Yanyang, Master candidate, Key Cell Engineering Laboratory, Affiliated Hospital of Zunyi Medical University, Zunyi 563000, Guizhou Province, China; Ministry of Tissue Damage Repair and Regenerative Medicine Jointly Established a Collaborative Innovation Center, Zunyi Medical University, Zunyi 563000, Guizhou Province, China -
Supported by:2022 National Natural Science Foundation (General Program), No. 32270848 (to HZX); 2020 Ministry of Education Collaborative Innovation Center Construction Project: Ministry of Tissue Damage Repair and Regenerative Medicine Jointly Established a Collaborative Innovation Center, No. [2020]39 (to HZX); 2020 Guizhou Provincial Science and Technology Support Plan Project, No. [2020]4Y192 (to HZX)
CLC Number:
Cite this article
Wang Yanyang, Liu Chan, Yu Limei, He Zhixu. Current status and future of treatment of pulmonary fibrosis by mesenchymal stem cells and extracellular vesicles[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(25): 4079-4086.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
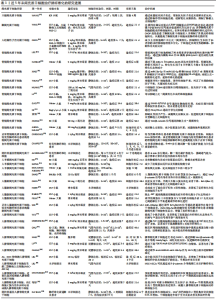
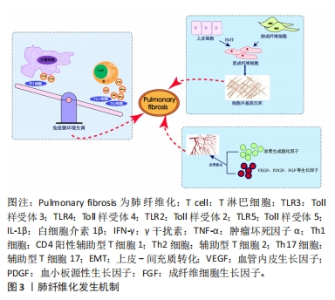
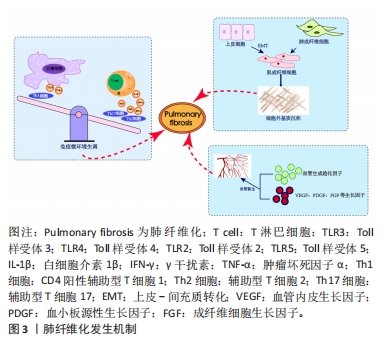
2.1.1 免疫微环境改变 肺纤维化最初被描述为一种以炎症反应为始的疾病[19],研究证明炎症反应几乎参与了所有的伤口愈合发生与纤维化进程[20],其中在纤维化进程中炎症微环境发生的主要变化包括:①免疫细胞群与炎症因子。新近研究通过单细胞测序技术揭示肺纤维化过程中免疫细胞群的主要改变以巨噬细胞与T细胞为主[21]。巨噬细胞分为促进炎症的M1型巨噬细胞和抗炎的M2型巨噬细胞,M1型巨噬细胞负责肺泡上皮损伤后的调节过程,分泌肿瘤坏死因子α等肺纤维化相关炎症因子。M2型巨噬细胞主要在伤口愈合过程、肺部炎症反应中发挥关键作用,分泌白细胞介素10等肺纤维化过程的重要因子。各种调节细胞因子、趋化因子、递质和免疫调节细胞通过改变M1、M2型巨噬细胞的极化过程来作用于肺部疾患,从而影响肺纤维化的发展过程[22-23]。在T细胞群中,Th1细胞参与吞噬细胞依赖性炎症,分泌大量白细胞介素2等,Th2细胞参与慢性炎症疾病和组织修复,分泌大量白细胞介素4等[24]。正常伤口有效愈合通常以占主导地位的Th1反应为特征,而免疫平衡转为以Th2细胞为主导会导致慢性炎症,最终导致纤维化[25],不平衡的Th1/Th2免疫反应被认为是特发性肺纤维化发病机制的核心。研究发现,Th17分化受阻可以减轻博莱霉素诱导的肺纤维化[24,26]。②Toll样受体与炎性小体等免疫活性物质。Toll样受体是参与非特异性免疫(天然免疫)的一类重要蛋白质分子,表达在巨噬细胞、树突状细胞和上皮细胞表面。研究表明,Toll样受体3、Toll样受体4缺乏会促进肺纤维化中异常的炎症反应和纤维增殖反应,Toll样受体2、Toll样受体5在肺纤维化动物模型中表达增加[27-30]。在肺纤维化发生中现有研究比较多的炎性小体是NLRP3与AIM2炎性小体,NLRP3在肺泡上皮细胞中被激活,可以通过转化生长因子β1调节上皮-间质转化参与肺纤维化进程[31-32]。CHO等[33]通过临床试验证明,AIM2炎性小体在特发性肺纤维化患者的外周血单核细胞中会增加,AIM2炎性小体激活有助于产生和释放促纤维化递质。 2.1.2 上皮、间质细胞群改变导致的间质沉积 肺纤维化过程中,肌成纤维细胞大量产生导致间质沉积的途径包括:①间质细胞种群发生变化:成纤维细胞向肌成纤维细胞的过渡是伤口愈合过程中的正常事件,其特征是平滑肌肌动蛋白达增加。肌成纤维细胞是产生闭合伤口收缩力所必需的要素,这与胶原蛋白沉积有关[34],肌成纤维细胞数量的增加会导致纤维化的进展,单细胞测序结果揭示了在肺纤维化发生发展中,肌成纤维细胞特异性表面标志物表达在平滑肌细胞等多种细胞中,所有间充质细胞中与细胞外基质相关的基因都上调[35]。②肺上皮细胞发生上皮-间质转化。临床样本与实验动物肺部组织病理染色中间充质与上皮细胞标志物的共定位证实了上皮-间质转化的发生[36-38] ,单细胞测序揭示肺纤维化肺上皮细胞的特点是气道上皮细胞比例增加,肺泡上皮细胞大幅下降,出现的异常基底细胞表达上皮-间质转化的标志物[21],肺纤维化过程中,转化生长因子β、血小板衍生生长因子受体、成纤维细胞生长因子和多种信号通路都能促进间充质基因的表达和上皮基因的下调[4,39]。 2.1.3 血管生成 血管生成是伤口修复的正常特征,能够提供组织愈合所需的细胞和营养。研究发现,在肺纤维化过程中,纤维化区域内出现广泛新生血管[40]。血管生成的稳态由血管生成促进和抑制因素共同调节,例如血管生成趋化因子5、血管生成趋化因子8与血管生成趋化因子10的表达不平衡,可以使血管生成增加,抑制血管生成趋化因子受体2可以减弱博莱霉素诱导的肺纤维化程度[41-42];也有研究证明血管生成素生物轴与肺纤维化血管重塑的调节明确相关[43]; 血管内皮生长因子、血小板衍生生长因子和成纤维细胞生长因子也都与肺纤维化的发病机制相关[44],血管内皮生长因子是最有效的血管生成刺激因素之一,在肺纤维化发生发展过程中发挥重要作用[45-46]。 2.2 MSCs治疗肺纤维化的研究进展 在中国知网、PubMed数据库筛选出近5年内涉及MSCs治疗肺纤维化的相关研究37篇[47-83],其中包括34个临床前研究,3个临床研究,研究所涉及的具体细节按照MSCs种类进行分类,造模方法、细胞移植途径、剂量、时间等具体细节见表1。关于MSCs治疗肺纤维化的机制,迄今为止研究最充分的是炎症改善,不同来源MSCs可以通过调整中性粒细胞比例、T细胞数量、Treg平衡来对抗炎症[47-50],现有的通路研究包含SMAD-3/TGF-β信号通路、PD-1/PD-L1通路、AKT通路、IL-6/IL-10/TGF-β轴、Wnt/β-catenin通路[51-55],通过不同的作用机制最终使得促炎因子下调,抗炎因子上调;其次是上皮间质细胞群的改善[56]:其中包括肺上皮细胞的凋亡减轻[57-59],成纤维细胞的激活或增殖被抑制[60],上皮-间质转化的改善[61],现有研究多数集中在通过Wnt/β-catenin信号通路减缓上皮-间质转化的发生[62-63];最后是氧化应激过程的改善[64-65]。大多数情况下,上述改变并不单一发生,多种机制发生综合作用[66],最终完成纤维化的改善。"
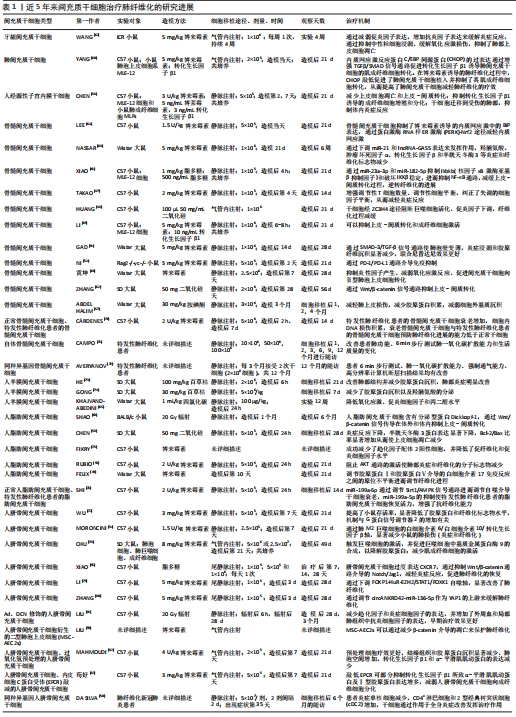
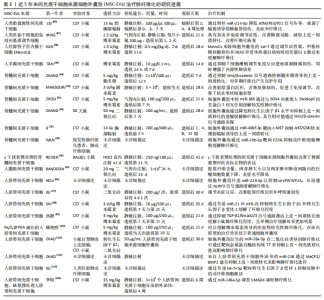
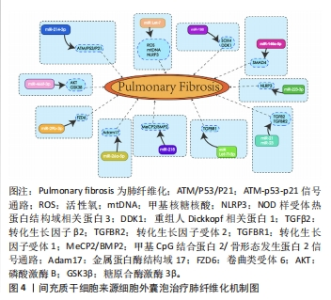
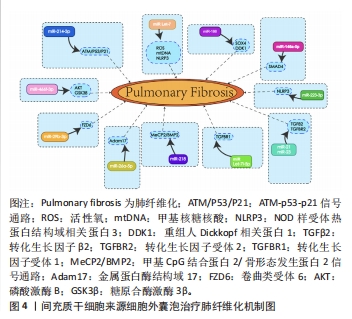
2.3 MSC-EVs治疗肺纤维化研究进展 在中国知网、PubMed数据库筛选近5年来涉及MSC-EVs治疗肺纤维化相关研究21篇[84-104],实验涉及的具体细节见表2,其作用机制主要通过细胞外囊泡介导的miRNA引起靶向通路改变进而产生抗纤维化效应,多数研究阐明具体发挥作用的miRNA及其靶基因,汇总图见图4,其作用的发挥也主要是通过抑制相关炎症因子,从而减轻肺部纤维病变,同时促进肺部组织修复。值得关注的是,较多研究着力于上皮-间质转化的延缓,其中较为重要的是不同来源的MSCs能通过Wnt/β-catenin信号通路的改变抑制上皮-间质转化的发生[84-85],至于其他研究,发挥具体作用的miRNA及其相应靶基因还有待进一步探讨。"

| [1] NALYSNYK L, CID-RUZAFA J, ROTELLA P, et al. Incidence and prevalence of idiopathic pulmonary fibrosis: review of the literature. Eur Respir Rev. 2012; 21(126):355-361. [2] KING TE JR, PARDO A, SELMAN M. Idiopathic pulmonary fibrosis. Lancet. 2011; 378(9807):1949-1961. [3] WAKWAYA Y, BROWN KK. Idiopathic Pulmonary Fibrosis: Epidemiology, Diagnosis and Outcomes. Am J Med Sci. 2019;357(5):359-369. [4] MOSS BJ, RYTER SW, ROSAS IO. Pathogenic Mechanisms Underlying Idiopathic Pulmonary Fibrosis. Annu Rev Pathol. 2022;17:515-546. [5] CHIOMA OS, DRAKE WP. Role of Microbial Agents in Pulmonary Fibrosis. Yale J Biol Med. 2017;90(2):219-227. [6] GENTILE F, AIMO A, FORFORI F, et al. COVID-19 and risk of pulmonary fibrosis: the importance of planning ahead. Eur J Prev Cardiol. 2020;27(13):1442-1446. [7] CAPLAN AI. Mesenchymal stem cells. J Orthop Res. 1991;9(5):641-650. [8] ANDRZEJEWSKA A, LUKOMSKA B, JANOWSKI M. Concise Review: Mesenchymal Stem Cells: From Roots to Boost. Stem Cells. 2019;37(7):855-864. [9] VASANTHAN J, GURUSAMY N, RAJASINGH S, et al. Role of Human Mesenchymal Stem Cells in Regenerative Therapy. Cells. 2020;10(1):54. [10] MISHRA VK, SHIH HH, PARVEEN F, et al. Identifying the Therapeutic Significance of Mesenchymal Stem Cells. Cells. 2020;9(5):1145. [11] SAMSONRAJ RM, RAGHUNATH M, NURCOMBE V, et al. Concise Review: Multifaceted Characterization of Human Mesenchymal Stem Cells for Use in Regenerative Medicine. Stem Cells Transl Med. 2017;6(12):2173-2185. [12] LI DY, LI RF, SUN DX, et al. Mesenchymal stem cell therapy in pulmonary fibrosis: a meta-analysis of preclinical studies. Stem Cell Res Ther. 2021;12(1):461. [13] LAI P, WENG J, GUO L, et al. Novel insights into MSC-EVs therapy for immune diseases. Biomark Res. 2019;7:6. [14] HARRELL CR, JOVICIC N, DJONOV V, et al. Mesenchymal Stem Cell-Derived Exosomes and Other Extracellular Vesicles as New Remedies in the Therapy of Inflammatory Diseases. Cells. 2019;8(12):1605. [15] RANI S, RYAN AE, GRIFFIN MD, et al. Mesenchymal Stem Cell-derived Extracellular Vesicles: Toward Cell-free Therapeutic Applications. Mol Ther. 2015;23(5):812-823. [16] FERNÁNDEZ-FRANCOS S, EIRO N, COSTA LA, et al. Mesenchymal Stem Cells as a Cornerstone in a Galaxy of Intercellular Signals: Basis for a New Era of Medicine. Int J Mol Sci. 2021;22(7):3576. [17] MEYER KC. Pulmonary fibrosis, part I: epidemiology, pathogenesis, and diagnosis. Expert Rev Respir Med. 2017;11(5):343-359. [18] WANG YY, ZHANG CY, MA YQ, et al. Therapeutic effects of C-28 methyl ester of 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid (CDDO-Me; bardoxolone methyl) on radiation-induced lung inflammation and fibrosis in mice. Drug Des Devel Ther. 2015;9:3163-3178. [19] AMERICAN THORACIC SOCIETY. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med. 2000; 161(2 Pt 1):646-664. [20] WICK G, GRUNDTMAN C, MAYERL C, et al. The immunology of fibrosis. Annu Rev Immunol. 2013;31:107-135. [21] ADAMS TS, SCHUPP JC, POLI S, et al. Single-cell RNA-seq reveals ectopic and aberrant lung-resident cell populations in idiopathic pulmonary fibrosis. Sci Adv. 2020;6(28):eaba1983. [22] LOCATI M, MANTOVANI A, SICA A. Macrophage activation and polarization as an adaptive component of innate immunity. Adv Immunol. 2013;120:163-184. [23] ZHANG L, WANG Y, WU G, et al. Macrophages: friend or foe in idiopathic pulmonary fibrosis? Respir Res. 2018;19(1):170. [24] HEUKELS P, MOOR CC, VON DER THÜSEN JH, et al. Inflammation and immunity in IPF pathogenesis and treatment. Respir Med. 2019;147:79-91. [25] WICK G, BACKOVIC A, RABENSTEINER E, et al. The immunology of fibrosis: innate and adaptive responses. Trends Immunol. 2010;31(3):110-119. [26] PARK SJ, HAHN HJ, OH SR, et al. Theophylline Attenuates BLM-Induced Pulmonary Fibrosis by Inhibiting Th17 Differentiation. Int J Mol Sci. 2023;24(2):1019. [27] LIANG J, ZHANG Y, XIE T, et al. Hyaluronan and TLR4 promote surfactant-protein-C-positive alveolar progenitor cell renewal and prevent severe pulmonary fibrosis in mice. Nat Med. 2016;22(11):1285-1293. [28] MILLIEN VO, LU W, SHAW J, et al. Cleavage of fibrinogen by proteinases elicits allergic responses through Toll-like receptor 4. Science. 2013;341(6147):792-796. [29] O’DWYER DN, ARMSTRONG ME, TRUJILLO G, et al. The Toll-like receptor 3 L412F polymorphism and disease progression in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2013;188(12):1442-1450. [30] MCELROY AN, INVERNIZZI R, LASKOWSKA JW, et al. Candidate Role for Toll-like Receptor 3 L412F Polymorphism and Infection in Acute Exacerbation of Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med. 2022;205(5):550-562. [31] TIAN R, ZHU Y, YAO J, et al. NLRP3 participates in the regulation of EMT in bleomycin-induced pulmonary fibrosis. Exp Cell Res. 2017;357(2):328-334. [32] JÄGER B, SEELIGER B, TERWOLBECK O, et al. The NLRP3-Inflammasome-Caspase-1 Pathway Is Upregulated in Idiopathic Pulmonary Fibrosis and Acute Exacerbations and Is Inducible by Apoptotic A549 Cells. Front Immunol. 2021; 12:642855. [33] CHO SJ, MOON JS, NIKAHIRA K, et al. GLUT1-dependent glycolysis regulates exacerbation of fibrosis via AIM2 inflammasome activation. Thorax. 2020;75(3): 227-236. [34] TOMASEK JJ, GABBIANI G, HINZ B, et al. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3(5):349-363. [35] TSUKUI T, SUN KH, WETTER JB, et al. Collagen-producing lung cell atlas identifies multiple subsets with distinct localization and relevance to fibrosis. Nat Commun. 2020;11(1):1920. [36] CHILOSI M, CALIÒ A, ROSSI A, et al. Epithelial to mesenchymal transition-related proteins ZEB1, β-catenin, and β-tubulin-III in idiopathic pulmonary fibrosis. Mod Pathol. 2017;30(1):26-38. [37] WILLIS BC, LIEBLER JM, LUBY-PHELPS K,et al. Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: potential role in idiopathic pulmonary fibrosis. Am J Pathol. 2005;166(5):1321-1332. [38] MARMAI C, SUTHERLAND RE, KIM KK, et al. Alveolar epithelial cells express mesenchymal proteins in patients with idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2011;301(1):L71-L78. [39] MAHER TM, OBALLA E, SIMPSON JK, et al. An epithelial biomarker signature for idiopathic pulmonary fibrosis: an analysis from the multicentre PROFILE cohort study. Lancet Respir Med. 2017;5(12):946-955. [40] BARRATT S, MILLAR A. Vascular remodelling in the pathogenesis of idiopathic pulmonary fibrosis. QJM. 2014;107(7):515-519. [41] KEANE MP, ARENBERG DA, LYNCH JP 3RD, et al. The CXC chemokines, IL-8 and IP-10, regulate angiogenic activity in idiopathic pulmonary fibrosis. J Immunol. 1997;159(3):1437-1443. [42] RUSSO RC, GUABIRABA R, GARCIA CC, et al. Role of the chemokine receptor CXCR2 in bleomycin-induced pulmonary inflammation and fibrosis. Am J Respir Cell Mol Biol. 2009;40(4):410-421. [43] MARGARITOPOULOS GA, ANTONIOU KM, KARAGIANNIS K, et al. Investigation of angiogenetic axis Angiopoietin-1 and -2/Tie-2 in fibrotic lung diseases: a bronchoalveolar lavage study. Int J Mol Med. 2010;26(6):919-923. [44] AMANO H, MATSUI Y, HATANAKA K, et al. VEGFR1-tyrosine kinase signaling in pulmonary fibrosis. Inflamm Regen. 2021;41(1):16. [45] ANDO M, MIYAZAKI E, ITO T, et al. Significance of serum vascular endothelial growth factor level in patients with idiopathic pulmonary fibrosis. Lung. 2010; 188(3):247-252. [46] MURRAY LA, HABIEL DM, HOHMANN M, et al. Antifibrotic role of vascular endothelial growth factor in pulmonary fibrosis. JCI Insight. 2017;2(16):e92192. [47] TAKAO S, NAKASHIMA T, MASUDA T, et al. Human bone marrow-derived mesenchymal stromal cells cultured in serum-free media demonstrate enhanced antifibrotic abilities via prolonged survival and robust regulatory T cell induction in murine bleomycin-induced pulmonary fibrosis. Stem Cell Res Ther. 2021;12(1):506. [48] HUANG J, HUANG J, NING X,et al. CT/NIRF dual-modal imaging tracking and therapeutic efficacy of transplanted mesenchymal stem cells labeled with Au nanoparticles in silica-induced pulmonary fibrosis. J Mater Chem B. 2020;8(8): 1713-1727. [49] FELIX RG, BOVOLATO ALC, COTRIM OS, et al. Adipose-derived stem cells and adipose-derived stem cell-conditioned medium modulate in situ imbalance between collagen I- and collagen V-mediated IL-17 immune response recovering bleomycin pulmonary fibrosis. Histol Histopathol. 2020;35(3):289-301. [50] DA SILVA KN, PINHEIRO PCG, GOBATTO ALN, et al. Immunomodulatory and Anti-fibrotic Effects Following the Infusion of Umbilical Cord Mesenchymal Stromal Cells in a Critically Ill Patient With COVID-19 Presenting Lung Fibrosis: A Case Report. Front Med (Lausanne). 2021;8:767291. [51] GAD ES, SALAMA AAA, EL-SHAFIE MF, et al. The Anti-fibrotic and Anti-inflammatory Potential of Bone Marrow-Derived Mesenchymal Stem Cells and Nintedanib in Bleomycin-Induced Lung Fibrosis in Rats. Inflammation. 2020; 43(1):123-134. [52] NI K, LIU M, ZHENG J, et al. PD-1/PD-L1 Pathway Mediates the Alleviation of Pulmonary Fibrosis by Human Mesenchymal Stem Cells in Humanized Mice. Am J Respir Cell Mol Biol. 2018;58(6):684-695. [53] RUBIO GA, ELLIOT SJ, WIKRAMANAYAKE TC, et al. Mesenchymal stromal cells prevent bleomycin-induced lung and skin fibrosis in aged mice and restore wound healing. J Cell Physiol. 2018;233(8):5503-5512. [54] MORONCINI G, PAOLINI C, ORLANDO F, et al. Mesenchymal stromal cells from human umbilical cord prevent the development of lung fibrosis in immunocompetent mice. PLoS One. 2018;13(6):e0196048. [55] XIAO K, LIU C, WANG H, et al. Umbilical cord mesenchymal stem cells overexpressing CXCR7 facilitate treatment of ARDS-associated pulmonary fibrosis via inhibition of Notch/Jag1 mediated by the Wnt/β-catenin pathway. Biomed Pharmacother. 2023;165:115124. [56] CHEN X, WU Y, WANG Y, et al. Human menstrual blood-derived stem cells mitigate bleomycin-induced pulmonary fibrosis through anti-apoptosis and anti-inflammatory effects. Stem Cell Res Ther. 2020;11(1):477. [57] ABDEL HALIM AS, AHMED HH, AGLAN HA, et al. Role of bone marrow-derived mesenchymal stem cells in alleviating pulmonary epithelium damage and extracellular matrix remodeling in a rat model of lung fibrosis induced by amiodarone. Biotech Histochem. 2021;96(6):418-430. [58] CHEN S, CUI G, PENG C, et al. Transplantation of adipose-derived mesenchymal stem cells attenuates pulmonary fibrosis of silicosis via anti-inflammatory and anti-apoptosis effects in rats. Stem Cell Res Ther. 2018;9(1):110. [59] LIU J, PENG D, YOU J, et al. Type 2 Alveolar Epithelial Cells Differentiated from Human Umbilical Cord Mesenchymal Stem Cells Alleviate Mouse Pulmonary Fibrosis Through β-Catenin-Regulated Cell Apoptosis. Stem Cells Dev. 2021; 30(13):660-670. [60] YANG X, SUN W, JING X, et al. Endoplasmic reticulum stress modulates the fate of lung resident mesenchymal stem cell to myofibroblast via C/EBP homologous protein during pulmonary fibrosis. Stem Cell Res Ther. 2022;13(1):279. [61] XIAO K, HE W, GUAN W, et al. Mesenchymal stem cells reverse EMT process through blocking the activation of NF-κB and Hedgehog pathways in LPS-induced acute lung injury. Cell Death Dis. 2020;11(10):863. [62] ZHANG E, YANG Y, CHEN S, et al. Bone marrow mesenchymal stromal cells attenuate silica-induced pulmonary fibrosis potentially by attenuating Wnt/β-catenin signaling in rats. Stem Cell Res Ther. 2018;9(1):311. [63] SHAO L, ZHANG Y, SHI W, et al. Mesenchymal stromal cells can repair radiation-induced pulmonary fibrosis via a DKK-1-mediated Wnt/β-catenin pathway. Cell Tissue Res. 2021;384(1):87-97. [64] LEE EJ, CÁRDENES N, ÁLVAREZ D, et al. Mesenchymal stem cells reduce ER stress via PERK-Nrf2 pathway in an aged mouse model. Respirology. 2020;25(4):417-426. [65] KHAJVAND-ABEDINI M, BAHMANI M, ZIAMAJIDI N, et al. The Restoring Effect of Human Umbilical Cord-Derived Mesenchymal Cell-Conditioned Medium (hMSC-CM) against Carbon Tetrachloride-Induced Pulmonary Fibrosis in Male Wistar Rats. Int J Inflam. 2022;2022:7179766. [66] 黄坤,周勇,刘美芳,等. 甘草酸二铵联合骨髓间充质干细胞治疗大鼠肺纤维化急性加重的实验研究[J].中国呼吸与危重监护杂志,2020,19(1):64-69. [67] WANG X, ZHAO S, LAI J, et al. Anti-Inflammatory, Antioxidant, and Antifibrotic Effects of Gingival-Derived MSCs on Bleomycin-Induced Pulmonary Fibrosis in Mice. Int J Mol Sci. 2021;23(1):99. [68] NASSAR SZ, ABDELMONSIF DA, ALI RG, et al. Sodium hydrosulfide and bone marrow derived mesenchymal stem cells combined therapy for bleomycin induced pulmonary fibrosis in rats: Implication of micro RNA-21 and Lnc GAS5. Life Sci. 2022;309:120988. [69] LI X, LI C, TANG Y, et al. NMDA receptor activation inhibits the antifibrotic effect of BM-MSCs on bleomycin-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2018;315(3):L404-L421. [70] CÁRDENES N, ÁLVAREZ D, SELLARÉS J, et al. Senescence of bone marrow-derived mesenchymal stem cells from patients with idiopathic pulmonary fibrosis. Stem Cell Res Ther. 2018;9(1):257. [71] CAMPO A, GONZÁLEZ-RUIZ JM, ANDREU E, et al. Endobronchial autologous bone marrow-mesenchymal stromal cells in idiopathic pulmonary fibrosis: a phase I trial. ERJ Open Res. 2021;7(2):00773-2020. [72] AVERYANOV A, KOROLEVA I, KONOPLYANNIKOV M, et al. First-in-human high-cumulative-dose stem cell therapy in idiopathic pulmonary fibrosis with rapid lung function decline. Stem Cells Transl Med. 2020;9(1):6-16. [73] HE F, WANG Y, LI Y, et al. Human amniotic mesenchymal stem cells alleviate paraquat-induced pulmonary fibrosis in rats by inhibiting the inflammatory response. Life Sci. 2020;243:117290. [74] GONG L, WANG X, XU S, et al. Human Amnion-Derived MSCs Alleviate Acute Lung Injury and Hinder Pulmonary Fibrosis Caused by Paraquat in Rats. Oxid Med Cell Longev. 2022;2022:3932070. [75] FIKRY H, SALEH LA, GAWAD SA. Therapeutic effect of adipose-derived mesenchymal stem cells (AD-MSCs) compared to pirfenidone on corticosteroid resistance in a mouse model of acute exacerbation of idiopathic pulmonary fibrosis. Histol Histopathol. 2022;37(11):1065-1083. [76] Shi L, Han Q, Hong Y, et al. Inhibition of miR-199a-5p rejuvenates aged mesenchymal stem cells derived from patients with idiopathic pulmonary fibrosis and improves their therapeutic efficacy in experimental pulmonary fibrosis. Stem Cell Res Ther. 2021;12(1):147. [77] WU X, GOU H, ZHOU O, et al. Human umbilical cord mesenchymal stem cells combined with pirfenidone upregulates the expression of RGS2 in the pulmonary fibrosis in mice. Respir Res. 2022;23(1):270. [78] CHU KA, WANG SY, YEH CC, et al. Reversal of bleomycin-induced rat pulmonary fibrosis by a xenograft of human umbilical mesenchymal stem cells from Wharton’s jelly. Theranostics. 2019;9(22):6646-6664. [79] LI R, ZHANG H, ZHANG J, et al. hucMSCs treatment ameliorated pulmonary fibrosis via downregulating the circFOXP1-HuR-EZH2/STAT1/FOXK1 autophagic axis. Stem Cells. 2023:sxad053. [80] ZHANG H, ZHU Q, JI Y, et al. hucMSCs treatment prevents pulmonary fibrosis by reducing circANKRD42-YAP1-mediated mechanical stiffness. Aging (Albany NY). 2023;15(12):5514-5534. [81] LIU D, KONG F, YUAN Y, et al. Decorin-Modified Umbilical Cord Mesenchymal Stem Cells (MSCs) Attenuate Radiation-Induced Lung Injuries via Regulating Inflammation, Fibrotic Factors, and Immune Responses. Int J Radiat Oncol Biol Phys. 2018;101(4):945-956. [82] MAHMOUDI T, ABDOLMOHAMMADI K, BASHIRI H, et al. Hydrogen Peroxide Preconditioning Promotes Protective Effects of Umbilical Cord Vein Mesenchymal Stem Cells in Experimental Pulmonary Fibrosis. Adv Pharm Bull. 2020;10(1):72-80. [83] 苟好. EPCR基因敲低的人脐带间充质干细胞治疗博来霉素所致小鼠肺纤维化及机制初探[D].重庆:重庆医科大学,2018. [84] ZHANG X, YE L, TANG W, et al. Wnt/β-Catenin Participates in the Repair of Acute Respiratory Distress Syndrome-Associated Early Pulmonary Fibrosis via Mesenchymal Stem Cell Microvesicles. Drug Des Devel Ther. 2022;16:237-247. [85] ZHANG E, GENG X, SHAN S, et al. Exosomes derived from bone marrow mesenchymal stem cells reverse epithelial-mesenchymal transition potentially via attenuating Wnt/β-catenin signaling to alleviate silica-induced pulmonary fibrosis. Toxicol Mech Methods. 2021;31(9):655-666. [86] LEI X, HE N, ZHU L, et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles Attenuate Radiation-Induced Lung Injury via miRNA-214-3p. Antioxid Redox Signal. 2021;35(11):849-862. [87] YANG S, LIU P, GAO T, et al. Every road leads to Rome: therapeutic effect and mechanism of the extracellular vesicles of human embryonic stem cell-derived immune and matrix regulatory cells administered to mouse models of pulmonary fibrosis through different routes. Stem Cell Res Ther. 2022;13(1):163. [88] SUN L, ZHU M, FENG W, et al. Exosomal miRNA Let-7 from Menstrual Blood-Derived Endometrial Stem Cells Alleviates Pulmonary Fibrosis through Regulating Mitochondrial DNA Damage. Oxid Med Cell Longev. 2019;2019:4506303. [89] TAN JL, LAU SN, LEAW B, et al. Amnion Epithelial Cell-Derived Exosomes Restrict Lung Injury and Enhance Endogenous Lung Repair. Stem Cells Transl Med. 2018; 7(2):180-196. [90] MANSOURI N, WILLIS GR, FERNANDEZ-GONZALEZ A, et al. Mesenchymal stromal cell exosomes prevent and revert experimental pulmonary fibrosis through modulation of monocyte phenotypes. JCI Insight. 2019;4(21):e128060. [91] ZHOU J, LIN Y, KANG X, et al. microRNA-186 in extracellular vesicles from bone marrow mesenchymal stem cells alleviates idiopathic pulmonary fibrosis via interaction with SOX4 and DKK1. Stem Cell Res Ther. 2021;12(1):96. [92] LI Y, SHEN Z, JIANG X, et al. Mouse mesenchymal stem cell-derived exosomal miR-466f-3p reverses EMT process through inhibiting AKT/GSK3β pathway via c-MET in radiation-induced lung injury. J Exp Clin Cancer Res. 2022;41(1):128. [93] WAN X, CHEN S, FANG Y, et al. Mesenchymal stem cell-derived extracellular vesicles suppress the fibroblast proliferation by downregulating FZD6 expression in fibroblasts via micrRNA-29b-3p in idiopathic pulmonary fibrosis. J Cell Physiol. 2020;235(11):8613-8625. [94] ROZIER P, MAUMUS M, MARIA ATJ, et al. Lung Fibrosis Is Improved by Extracellular Vesicles from IFNγ-Primed Mesenchymal Stromal Cells in Murine Systemic Sclerosis. Cells. 2021;10(10):2727. [95] BANDEIRA E, OLIVEIRA H, SILVA JD, et al. Therapeutic effects of adipose-tissue-derived mesenchymal stromal cells and their extracellular vesicles in experimental silicosis. Respir Res. 2018;19(1):104. [96] HOU L, ZHU Z, JIANG F, et al. Human umbilical cord mesenchymal stem cell-derived extracellular vesicles alleviated silica induced lung inflammation and fibrosis in mice via circPWWP2A/miR-223-3p/NLRP3 axis. Ecotoxicol Environ Saf. 2023;251:114537. [97] XU C, ZHAO J, LI Q, et al. Exosomes derived from three-dimensional cultured human umbilical cord mesenchymal stem cells ameliorate pulmonary fibrosis in a mouse silicosis model. Stem Cell Res Ther. 2020;11(1):503. [98] SHI L, REN J, LI J, et al. Extracellular vesicles derived from umbilical cord mesenchymal stromal cells alleviate pulmonary fibrosis by means of transforming growth factor-β signaling inhibition. Stem Cell Res Ther. 2021;12(1):230. [99] 杨静,胡华钟,张书勤,等.脐带间充质干细胞来源的外泌体通过抑制上皮间质转化缓解肺纤维化[J].南方医科大学学报,2020,40(7):988-994. [100] 韩艳煦. 磁化脐带间充质干细胞外泌体对特发性肺纤维化的治疗研究[D].长春:东北师范大学,2021. [101] ZHAO J, JIANG Q, XU C, et al. MiR-26a-5p from HucMSC-derived extracellular vesicles inhibits epithelial mesenchymal transition by targeting Adam17 in silica-induced lung fibrosis. Ecotoxicol Environ Saf. 2023;257:114950. [102] ZHAO Y, DU L, SUN J, et al. Exosomal miR-218 derived from mesenchymal stem cells inhibits endothelial-to-mesenchymal transition by epigenetically modulating of BMP2 in pulmonary fibrosis. Cell Biol Toxicol. 2023. doi: 10.1007/s10565-023-09810-z. [103] XU C, HOU L, ZHAO J, et al. Exosomal let-7i-5p from three-dimensional cultured human umbilical cord mesenchymal stem cells inhibits fibroblast activation in silicosis through targeting TGFBR1. Ecotoxicol Environ Saf. 2022;233:113302. [104] 李晗.缺氧预处理人脐带间充质干细胞来源外泌体通过miRNA-146a-5p调控SMAD4减轻肺纤维化的研究[D].长春:吉林大学,2023. [105] FURLANI D, UGURLUCAN M, ONG L, et al. Is the intravascular administration of mesenchymal stem cells safe? Mesenchymal stem cells and intravital microscopy. Microvasc Res. 2009;77(3):370-376. [106] WEISS ARR, DAHLKE MH. Immunomodulation by Mesenchymal Stem Cells (MSCs): Mechanisms of Action of Living, Apoptotic, and Dead MSCs. Front Immunol. 2019;10:1191. [107] DJOUAD F, PLENCE P, BONY C, et al Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood. 2003;102(10): 3837-3844. [108] SIRITHAMMAJAK S, MANOCHANTR S, TANTRAWATPAN C, et al. Human Mesenchymal Stem Cells Derived from the Placenta and Chorion Suppress the Proliferation while Enhancing the Migration of Human Breast Cancer Cells. Stem Cells Int. 2022;2022:4020845. [109] HU C, ZHAO L, ZHANG L, et al. Mesenchymal stem cell-based cell-free strategies: safe and effective treatments for liver injury. Stem Cell Res Ther. 2020;11(1):377. [110] ZHANG H, XIAO B, JIANG L, et al. Inhibition of mesenchymal stromal cells’ chemotactic effect to ameliorate paraquat-induced pulmonary fibrosis. Toxicol Lett. 2019;307:1-10. [111] LAN YW, CHOO KB, CHEN CM, et al. Hypoxia-preconditioned mesenchymal stem cells attenuate bleomycin-induced pulmonary fibrosis. Stem Cell Res Ther. 2015;6(1):97. [112] LV Y, YU C, LI X, et al. ROS-activatable nanocomposites for CT imaging tracking and antioxidative protection of mesenchymal stem cells in idiopathic pulmonary fibrosis therapy. J Control Release. 2023;357:249-263. |
| [1] | Yu Weijie, Liu Aifeng, Chen Jixin, Guo Tianci, Jia Yizhen, Feng Huichuan, Yang Jialin. Advantages and application strategies of machine learning in diagnosis and treatment of lumbar disc herniation [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1426-1435. |
| [2] | Chen Kaijia, Liu Jingyun, Cao Ning, Sun Jianbo, Zhou Yan, Mei Jianguo, Ren Qiang. Application and prospect of tissue engineering in treatment of osteonecrosis of the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1450-1456. |
| [3] | Lin Zeyu, Xu Lin. Research progress in gout-induced bone destruction mechanism [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1295-1300. |
| [4] | Feng Ruiqin, Han Na, Zhang Meng, Gu Xinyi, Zhang Fengshi. Combination of 1% platelet-rich plasma and bone marrow mesenchymal stem cells improves the recovery of peripheral nerve injury [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 985-992. |
| [5] | Qiu Xiaoyan, Li Bixin, Li Jingdi, Fan Chuiqin, Ma Lian, Wang Hongwu. Differentiation of insulin-producing cells from human umbilical cord mesenchymal stem cells infected by MAFA-PDX1 overexpressed lentivirus [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1000-1006. |
| [6] | Liu Qiwei, Zhang Junhui, Yang Yuan, Wang Jinjuan. Role and mechanism of umbilical cord mesenchymal stem cells on polycystic ovary syndrome [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1015-1020. |
| [7] | Liu Jianhong, Liao Shijie, Li Boxiang, Tang Shengping, Wei Zhendi, Ding Xiaofei. Extracellular vesicles carrying non-coding RNA regulate the activation of osteoclasts [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1076-1082. |
| [8] | Pan Xiaolong, Fan Feiyan, Ying Chunmiao, Liu Feixiang, Zhang Yunke. Effect and mechanism of traditional Chinese medicine on inhibiting the aging of mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1091-1098. |
| [9] | Liu Hanfeng, Wang Jingjing, Yu Yunsheng. Artificial exosomes in treatment of myocardial infarction: current status and prospects [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1118-1123. |
| [10] | Zhuge Xiaoxuan, Li Ce, Bao Guangjie, Kang Hong. Potential value of canonical and non-canonical roles of connexin 43 in disease treatment [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1130-1136. |
| [11] | Ma Shuwei, He Sheng, Han Bing, Zhang Liaoyun. Exosomes derived from mesenchymal stem cells in treatment of animals with acute liver failure: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1137-1142. |
| [12] | Zhang Kefan, Shi Hui. Research status and application prospect of cytokine therapy for osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(6): 961-967. |
| [13] | Li Jiaqi, Huang Yuanli, Li Yan, Wang Chunren, Han Qianqian. Mechanism and influencing factors in molecular weight degradation of non-cross-linked hyaluronic acid [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 747-752. |
| [14] | Xu Rong, Wang Haojie, Geng Mengxiang, Meng Kai, Wang Hui, Zhang Keqin, Zhao Huijing. Research advance in preparation and functional modification of porous polytetrafluoroethylene artificial blood vessels [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 759-765. |
| [15] | Chen Xiaofang, Zheng Guoshuang, Li Maoyuan, Yu Weiting. Preparation and application of injectable sodium alginate hydrogels [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 789-794. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||