Chinese Journal of Tissue Engineering Research ›› 2022, Vol. 26 ›› Issue (8): 1266-1271.doi: 10.12307/2022.234
Previous Articles Next Articles
Research progress of pulsed electromagnetic field in the treatment of postmenopausal osteoporosis
Xiao Hao1, 2, Liu Jing1, 2, Zhou Jun1, 2
- 1Department of Rehabilitation Medicine, 2Laboratory of Rehabilitation Medicine, The First Affiliated Hospital of University of South China, Hengyang 421001, Hunan Province, China
-
Received:2020-12-01Revised:2020-12-05Accepted:2021-01-16Online:2022-03-18Published:2021-11-02 -
Contact:Zhou Jun, MD, Chief physician, Associate professor, Master’s supervisor, Department of Rehabilitation Medicine, The First Affiliated Hospital of University of South China, Hengyang 421001, Hunan Province, China; Laboratory of Rehabilitation Medicine, The First Affiliated Hospital of University of South China, Hengyang 421001, Hunan Province, China -
About author:Xiao Hao, Master candidate, Department of Rehabilitation Medicine, The First Affiliated Hospital of University of South China, Hengyang 421001, Hunan Province, China -
Supported by:the National Natural Science Foundation of China, No. 81973917 (to ZJ); the Natural Science Foundation of Hunan Province, No. 2020JJ4085 (to ZJ)
CLC Number:
Cite this article
Xiao Hao, Liu Jing, Zhou Jun. Research progress of pulsed electromagnetic field in the treatment of postmenopausal osteoporosis[J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1266-1271.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
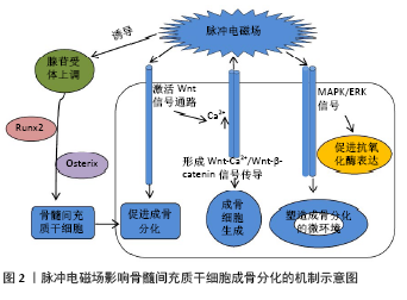
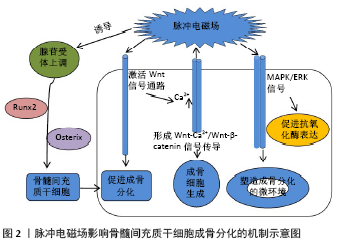
2.1 脉冲电磁场对绝经后骨质疏松症的作用 2.1.1 脉冲电磁场对骨密度的影响 骨密度是衡量骨强度的重要指标,也是诊断绝经后骨质疏松症及预测骨折危险性的重要依据。绝经后骨质疏松症治疗的关键是增加骨密度,抑制骨质流失,延缓骨质疏松症的进展。脉冲电磁场是增加绝经后骨质疏松患者骨密度、维持骨健康的重要举措。学者通过对绝经后妇女的腰椎(L2-L4)、股骨颈、股骨转子、髋部Ward三角等处的骨密度及骨矿物质含量的测定发现,脉冲电磁场治疗对骨密度的提升效应与负重运动疗效相当[11]。其次,当脉冲电磁场与抗骨质疏松药物联用时,在提升骨密度、改善预后方面发挥最大效应:脉冲电磁场与抗骨质疏松药物伊班膦酸钠(Ibandronate)联合运用时,发现伊班膦酸钠组、脉冲电磁场组和伊班膦酸钠+脉冲电磁场组大鼠L5椎体的骨密度增长比分别为21.6%、19.5%和39.6%[12],在增加骨密度方面具有显著的协同效应。绝经后骨质疏松症发病隐匿,进展迅速,脉冲电磁场治疗开始的时机是影响疗效的重要方面,体内实验研究提示脉冲电磁场在卵巢切除术后立即干预能有效提升腰椎(L5)、股骨的骨密度,而术后12周后干预仅仅只能有效抑制腰椎骨密度降低[13],提示干预时机与治疗效应呈正相关性,但类似的研究缺乏,急需更多有价值的数据证实。脉冲电磁场具有刺激频率或时间依赖的窗口效应,不同刺激参数可能导致不同疗效,当前临床试验研究的样本量以及临床试验方法设计的差异较大,尚不足以支持不同参数临床效应的验证,今后,需要更多高质量、多中心的临床研究系统探索脉冲电磁场的刺激参数的效应最大化。 2.1.2 脉冲电磁场对骨转化标志物的影响 绝经后骨质疏松症患者的骨转换标志物有助于评估合成代谢以及骨吸收状态,也是评估骨质疏松症治疗疗效的客观证据,同时对骨质疏松症的发生发展具有一定的预警效应。绝经后骨质疏松症患者的骨形成与骨吸收失平衡是导致骨小梁和皮质骨丢失、骨密度下降的根本原因。成骨细胞在增殖期主要表达Ⅰ型胶原羧基末端前肽(PICP),基质成熟期表达骨碱性磷酸酶,而矿化期间则主要表达骨钙素[14]。研究报道,脉冲电磁场可提高骨形成生物标志物水平,如骨钙素和Ⅰ型胶原羧基末端前肽,在增殖期和矿化期发挥增加骨形成单元数量、促进骨形成的作用[15]。另外,脉冲电磁场不仅可直接降低破骨细胞相关的骨吸收标志物,延缓骨吸收,如Ⅰ型胶原蛋白C末端交联肽(CTX)水平[16]、抗酒石酸酸性磷酸酶(TRACP5b)等[12,17],还能通过抑制肿瘤坏死因子α、白细胞介素1β和白细胞介素6等炎症因子水平抑制破骨细胞形成、分化及骨吸收功能[18],维持骨形成的正平衡状态[19]。 2.1.3 脉冲电磁场对骨组织形态及生物力学性能的影响 骨强度不仅取决于骨量的多少,更多的是依赖于骨内部结构的空间构筑,骨组织形态是承载和分散应力负荷的关键因素,也是体现治疗疗效的关键方面。脉冲电磁场在早期即可增加卵巢切除术鼠的骨小梁数目及骨小梁面积[20];何成奇等[21]分别用脉冲电磁场以20,40,60 min/d干预卵巢切除术鼠,检测发现3组均能提高股骨结构力学性能 (最大位移、载荷及能量吸收)和材料力学性能 (最大应力、应变及弹性模量),疗效相当,提示在20-60 min的时间段均为有效干预时间,为探索脉冲电磁场的干预参数提供了一定的参考依据。LEI等[22]使用低频、中频、高频的脉冲电磁场(1-100 Hz; 100-3 000 Hz;3 000-50 000 Hz)干预去卵巢大鼠8周后发现,3种不同频率的脉冲电磁场均能有效提升骨的显微结构及生物力学性能。脉冲电磁场对改善骨生物力学具有积极影响,遗憾的是目前缺乏更多有层次的多参数的实验研究,尚不能确定疗效最大化的参数范围,因此做更多相关类研究去探索治疗参数指导临床治疗是今后发展的必经之路。 2.1.4 脉冲电磁场对功能及预后的影响 绝经后骨质疏松症患者常伴有疼痛、平衡功能障碍、活动受限等功能障碍,伴随骨折高风险和持续高跌倒发生率,治疗骨质疏松症的最终目的是改善功能预后。一项随机对照试验评估脉冲电磁场治疗对58例骨质疏松症患者腰背部疼痛的疗效时发现,疼痛目测类比评分(VAS)均值从治疗前的6.97±1.83降至3.36±1.69,在减轻疼痛方面总体有效率高达93%[23]。此外,脉冲电磁场治疗能有效降低患者站立位时的前后摆幅指数、矩形面积和轨迹长度[24],增加行走时步幅、步速,降低双支撑相,是降低跌倒风险的有效治疗措施[25-26]。GIUSTI 等[26]通过使用便携式嵌入传感器系统评估脉冲电磁场治疗266例绝经后骨质疏松症患者的步态分析发现:通过6次脉冲电磁场治疗后患者的平均步速从(73.9±31.5) cm/s提高到了(94.1±25.5) cm/s,对提高步行能力疗效显著。骨质疏松症患者往往因长时程的病痛、生活自理能力下降等因素伴有抑郁症或抑郁样的心理改变[27],一项横断面研究采用患者健康问卷[The Patient Health Questionnaire (PHQ9)]评估绝经后骨质疏松症患者的抑郁程度,发现生活能力下降、疼痛是抑郁症的独立危险因素[28],提示有效减轻疼痛、提升日常生活能力是早期防治心理疾病发生发展的管控手段,脉冲电磁场在改善日常生活能力、提升生活质量等方面也有不可忽视的作用[29],在早期防治相应并发症方面具有一定的潜力,但目前对于探索脉冲电磁场对远期预后的评估缺乏大数据多样本的研究,可以作为今后研究的方向。 2.2 脉冲电磁场治疗绝经后骨质疏松症的相关机制研究 2.2.1 脉冲电磁场对骨髓间充质干细胞的机制研究 骨髓间充质干细胞是一种具有自我更新和多向分化能力的多能干细胞,在特定的诱导因子作用下可发生成骨分化,是维持骨形成、骨重建以及保持骨量必不可少的因素,如何诱导并增强骨髓间充质干细胞发生成骨分化是当前治疗骨质疏松症的新的靶点。研究证实脉冲电磁场干预2周时即可促进骨髓间充质干细胞增殖以及成骨相关基因和蛋白表达增加[30],其中低频率刺激更有利于细胞增殖,高频率刺激更倾向于成骨分化及骨重塑[31]。另外,当脉冲电磁场与骨形态发生蛋白联合干预骨髓间充质干细胞时发现脉冲电磁场不仅可增强成骨分化,而且具有充当促进骨髓间充质干细胞成骨分化的生物活性诱导因子的潜能[32]。脉冲电磁场通过多种作用机制影响骨髓间充质干细胞的成骨分化,研究显示脉冲电磁场可诱导腺苷受体上调,促进 Runx2和Osterix等成骨相关基因的表达介导骨髓间充质干细胞成骨分化[33]。此外,脉冲电磁场可通过诱导C3H10T1/2间充质细胞Ca2+内移以及核膜去极化,导致β-catenin易位至细胞核,激活经典的Wnt信号通路促进成骨分化;而Wnt结合到膜受体上后可激活L型和IP3敏感的钙通道,进一步导致胞内Ca2+增多,从而形成Wnt-Ca2+/Wnt-β-catenin信号传导途径诱导成骨细胞生成[34]。骨髓间充质干细胞成骨分化过程中呼吸蛋白酶亚单位增加、线粒体代谢上调[35],脉冲电磁场可通过MAPK/ERK信号促进抗氧化酶的表达[30],诱导细胞保护酶活性增强[36],塑造促进成骨分化的微环境,见图2。线粒体代谢介导骨髓间充质干细胞成骨分化是脉冲电磁场影响机制的前瞻性研究,是今后可以探索的方向。 自噬具有促进骨组织再生、维持骨稳态的潜能[37],在卵巢切除术鼠的骨髓间充质干细胞中Becin1蛋白表达减少,LC3II/LC3I比值降低,成骨分化和增殖能力明显下降,脉冲电磁场通过阻断mTOR相关蛋白途径提高骨髓间充质干细胞自噬水平,减轻骨质疏松症[38-39],目前脉冲电磁场对骨髓间充质干细胞自噬的影响研究只停留在体外阶段,缺乏体内实验验证,且其具体作用机制有待进一步研究。研究显示,炎症因子通过作用于骨髓间充质干细胞及细胞微环境,诱导细胞发生定向成脂分化是导致骨密度进行性下降的重要因素,并且炎症因子升高水平与松质骨骨密度下降程度成正相关[40],促进了骨质疏松症的发展进程,因此,靶向抑制骨髓间充质干细胞相关炎症通路是阻断骨质疏松症进展的重要机制。SIRT1与骨代谢、骨密度密切相关,研究发现,SIRT1能诱导成骨细胞特异性基因表达,并抑制过氧化物酶体增殖物激活受体γ(PPAP-γ) 和核因子kB 活性抑制骨髓间充质干细胞成脂分化,同时还通过抑制 NLRP3 炎症小体激活间接调控骨髓间充质干细胞分化[41- 42],而脉冲电磁场能否通过SIRT1调控NLRP3炎症小体信号抑制骨髓间充质干细胞焦亡发挥抗骨质疏松作用具有重要的探索价值。 2.2.2 脉冲电磁场对成骨细胞的机制研究 成骨细胞不仅是骨形成的直接承担者,而且可通过多种方式调节破骨细胞的增殖与分化,所以成骨细胞是防治骨质疏松症的关键,而脉冲电磁场对成骨细胞的影响是复杂多效的。脉冲电磁场可通过上调血清雌二醇和转化生长因子β水平[43]、增加成骨细胞因子活性[44]、促进转录因子受体的表达等途径促进成骨分化活性[45]。然而,CHANG等[46]发现当脉冲电磁场刺激颅骨成骨细胞碱性磷酸酶活性却降低,产生不同结论的原因可能是刺激参数不同以及干预分析的时间点不同。此外,脉冲电磁场还能通过下调细胞外基质降解相关基因,如基质金属蛋白酶11和DUSP4[47],以及增加胰岛素样生长因子1、转化生长因子β分泌的方式,促进细胞外基质合成,调节成骨细胞生长和代谢。脉冲电磁场的不同脉冲波对成骨细胞增殖分化的效应也有差异:ZHOU等[48]分析正弦、三角形、方形和锯齿形波对成骨细胞增殖、分化的影响时,发现方形电磁场可以促进成骨细胞的增殖,但无益于成骨分化;正弦电磁场抑制了细胞增殖,但增强了成骨分化;三角形电磁场不会影响细胞增殖,但却可诱导最强的成骨活性。Wnt/β-catenin 信号通路在促进成骨分化及维持骨量中具有关键作用,是防治骨质疏松症的关键靶点[49],受到广大科研学者的推崇。 脉冲电磁场通过提高Wnt信号通路相关基因的表达,如Wnt1a,Wnt3a,Lrp5和Lrp6[50],促进骨形成。一氧化氮具有调节细胞间的信息传递、影响组织血运等作用,少量的一氧化氮可以促进成骨细胞增殖分化和骨代谢[51],脉冲电磁场依赖于NO/cGMP信号途径介导Ⅰ型胶原和Osterix等成骨基因表达,促进成骨细胞成熟与矿化[52],当前这一研究机制仍处于早期探索阶段,值得开展更多研究去探索。环磷酸腺苷(cAMP)-蛋白激酶A(PKA)信号系统是负责促进骨形成所需的重要途径,PKA激活后可催化亚基磷酸化并发生核转移,引起CREB磷酸化激活转录因子c-fos和c-jun家族,诱导Runx-2和骨钙素在其启动子区域识别DNA结合位点[53],脉冲电磁场可直接或间接激活成骨细胞的sAC-cAMP-PKA-CREB信号通路,促进体外骨形成[54],目前少许体外实验已对此有前瞻性的研究,是今后科研挖掘的方向。长链非编码RNA(long non-coding RNA,lncRNA)在调控表观遗传、细胞分化等方面至关重要[55]。YANG等[56]发现在骨质疏松症患者及卵巢切除术鼠中均有lncRNA-ORLNC1过表达,体外实验证实lncRNA-ORLNC1-miR-296-Pten轴是介导骨髓间充质干细胞分化的关键调控因子;lncRNA ODSM-miR-139-3p-ELK1轴的表达抑制成骨细胞凋亡并促进成骨矿化能力[57],以上研究结果证实lncRNA是治疗骨质疏松症的潜在靶点,这为脉冲电磁场能否通过lncRNA途径影响成骨分化走向提供了新思路。 2.2.3 脉冲电磁场对破骨细胞的机制研究 破骨细胞是负责骨吸收的唯一细胞,是当前抗骨质疏松症药物的重要靶点。脉冲电磁场对破骨细胞生成、凋亡以及骨吸收功能均有影响。脉冲电磁场不仅可以通过直接减少破骨细胞数量[58]、下调TRAP和CTSK等破骨相关基因表达[59]、降低肿瘤坏死因子α、白细胞介素1β等炎症因子水平抑制破骨细胞形成[60]、分化以及骨吸收功能[19],而且可以通过抑制破骨细胞转录因子活性如活化T细胞核因子(Nuclear factor-activated T cell 1,NFATc1)、遏制Ca2+发生核转移等方式影响破骨细胞的分化成熟[61-62]。研究揭示不同频率的脉冲电磁场可通过不同的作用途径产生不同的效应:低频刺激主要通过激活ERK和p38 MAPK通路增强破骨细胞分化和活性,而高频刺激则是通过抑制核因子κB受体活化因子配体诱导的IkB磷酸化抑制破骨细胞分化,降低骨吸收能力[63],由于缺乏临床试验验证,不同参数的影响机制研究目前只停留在理论阶段,今后需要通过临床验证挖掘更多有价值的数据。此外,LEE等[64]发现缺氧诱导因子2α(HIF-2α)可通过直接调节TWIST2或TRAF6抑制成骨分化,驱动破骨细胞生成,还可以调节核因子κB受体活化因子配体的水平参与骨代谢,也为今后研究提供了参考意义。 2.2.4 脉冲电磁场对骨细胞的机制研究 骨细胞约占成人骨细胞总数的95%,在稳定血钙平衡及维持骨新陈代谢方面具有重要作用。骨细胞是骨骼中的机械感受器,相邻骨细胞的突起相互连接形成广泛而复杂的骨细胞网络,影响骨形成及骨吸收,故了解脉冲电磁场对骨细胞的反应机制,是脉冲电磁场调控骨代谢、维持骨健康的重要方面。骨细胞起源于成骨细胞,可表达成骨细胞特异性转录因子调节成骨分化活性,脉冲电磁场通过增强骨细胞基因表达、抑制细胞凋亡以及调节骨细胞网络分布等参与调节成骨分化[65]。骨细胞多途径影响破骨细胞成熟、活性及骨吸收功能:首先,骨细胞间的信号传递直接调节破骨细胞活动,比如间隙连接蛋白43(CX43)、IFN-β;其次,骨细胞通过分泌细胞因子介导破骨细胞活动,正常情况下骨细胞分泌骨保护素调控破骨细胞分化成熟;而凋亡的骨细胞则产生吸引破骨细胞前体和/或刺激破骨细胞发育的分子信号,如破骨细胞趋化因子高迁移率族蛋白B1(HMGB1),并上调核因子κB受体活化因子配体、肿瘤坏死因子、白细胞介素6等水平募集破骨细胞[65-66]。为了平衡骨形成和骨吸收,成熟的骨细胞一方面可选择性激活β-catenin及增加 Notch靶基因转录,促进骨形成,另一方面可通过产生Wnt通路的抑制因子,如DKK1、sFRP1和硬化蛋白,负向调控成骨分化。当前关于脉冲电磁场对骨细胞的影响机制研究甚少,更系统更准确地了解对靶细胞的作用机制可成为治疗骨质疏松症新的靶点。 "
| [1] 中华医学会物理医学与康复学分会,中国老年学和老年医学学会骨质疏松康复分会.原发性骨质疏松症康复干预中国专家共识[J].中华物理医学与康复杂志,2019,41(1):1-7. [2] 中国骨质疏松症流行病学调查及“健康骨骼”专项行动结果发布[J].中华骨质疏松和骨矿盐疾病杂志,2019,12(4):317-318. [3] ZHU S, HE H, ZHANG C, et al. Effects of pulsed electromagnetic fields on postmenopausal osteoporosis. Bioelectromagnetics. 2017;38(6):406-424. [4] BYUN DW, MOON SH, KIM T, et al.Assessment of patient-reported outcomes (PROs): treatment satisfaction, medication adherence, and quality of life (QoL) and the associated factors in postmenopausal osteoporosis (PMO) patients in Korea. J Bone Miner Metab. 2019;37(3): 563-572. [5] IMAZ I, ZEGARRA P, GONZÁLEZ-ENRÍQUEZ J, et al.Poor bisphosphonate adherence for treatment of osteoporosis increases fracture risk: systematic review and meta-analysis.Osteoporos Int. 2010;21(11):1943-1951. [6] MASSARI L, BENAZZO F, FALEZ F, et al.Biophysical stimulation of bone and cartilage: state of the art and future perspectives.Int Orthop. 2019; 43(3):539-551. [7] TONG J, SUN L, ZHU B, et al.Pulsed electromagnetic fields promote the proliferation and differentiation of osteoblasts by reinforcing intracellular calcium transients. Bioelectromagnetics. 2017;38(7):541-549. [8] CHALIDIS B, SACHINIS N, ASSIOTIS A, et al.Stimulation of bone formation and fracture healing with pulsed electromagnetic fields: biologic responses and clinical implications.Int J Immunopathol Pharmacol. 2011;24(1 Suppl 2):17-20. [9] QIU XS, LI XG, CHEN YX. Pulsed electromagnetic field (PEMF): A potential adjuvant treatment for infected nonunion. Med Hypotheses. 2020;136:109506. [10] 徐明义,张平.骨质疏松症的物理治疗研究进展[J].中国骨质疏松杂志,2017,23(9):1245-1249. [11] IWASA K, REDDI AH. Pulsed Electromagnetic Fields and Tissue Engineering of the Joints. Tissue Eng Part B Rev. 2018;24(2):144-154. [12] ZHOU J, LIAO Y, XIE H, et al. Effects of combined treatment with ibandronate and pulsed electromagnetic field on ovariectomy-induced osteoporosis in rats. Bioelectromagnetics. 2017;38(1):31-40. [13] ZHOU J, LIAO Y, ZENG Y, et al. Effect of intervention initiation timing of pulsed electromagnetic field on ovariectomy-induced osteoporosis in rats. Bioelectromagnetics. 2017;38(6):456-465. [14] EASTELL R, SZULC P. Use of bone turnover markers in postmenopausal osteoporosis. Lancet Diabetes Endocrinol. 2017;5(11):908-923. [15] SPADARO JA, SHORT WH, SHEEHE PR, et al. Electromagnetic effects on forearm disuse osteopenia: a randomized, double-blind, sham-controlled study. Bioelectromagnetics. 2011;32(4):273-282. [16] YOUM YH, GRANT RW, MCCABE LR, et al. Canonical Nlrp3 inflammasome links systemic low-grade inflammation to functional decline in aging. Cell metabolism. 2013;18(4):519-532. [17] SHAO X, YAN Z, WANG D, et al. Pulsed electromagnetic fields ameliorate skeletal deterioration in bone mass, microarchitecture and strength by enhancing canonical Wnt signaling-mediated bone formation in rats with spinal cord injury. J Neurotrauma. 2021 Jan 8. doi: 10.1089/neu.2020.7296. [18] YOKOTA K, SATO K, MIYAZAKI T, et al. Combination of tumor necrosis factor α and interleukin-6 induces mouse osteoclast-like cells with bone resorption activity both in vitro and in vivo. Arthritis Rheumatol. 2014;66(1):121-129. [19] CHANG K, HONG-SHONG CHANG W, YU YH, et al. Pulsed electromagnetic field stimulation of bone marrow cells derived from ovariectomized rats affects osteoclast formation and local factor production. Bioelectromagnetics. 2004;25(2):134-141. [20] OLTEAN-DAN D, DOGARU GB, APOSTU D, et al. Enhancement of bone consolidation using high-frequency pulsed electromagnetic fields (HF-PEMFs): An experimental study on rats. Bosn J Basic Med Sci. 2019;19(2):201-209. [21] 何成奇, 王维, 肖登, 等.不同治疗时间的脉冲电磁场对去势大鼠股骨生物力学性能的影响[J].中华物理医学与康复杂志,2007, 29(8):510-513. [22] LEI T, LI F, LIANG Z, et al. Effects of four kinds of electromagnetic fields (EMF) with different frequency spectrum bands on ovariectomized osteoporosis in mice. Sci Rep. 2017;7(1):553. [23] 谢小波, 崔红岩, 庞丽云,等.脉冲电磁场用于治疗骨质疏松性疼痛的疗效评估及分析[J].国际生物医学工程杂志,2011,14(2):107-110. [24] 何红晨, 徐健, 杨霖,等.低频脉冲电磁场对绝经后骨质疏松症患者平衡功能的影响[J].四川大学学报(医学版),2014,45(1):116-119. [25] GIUSTI A, GIOVALE M, PONTE M, et al. Short-term effect of low-intensity, pulsed, electromagnetic fields on gait characteristics in older adults with low bone mineral density: a pilot randomized-controlled trial. Geriatr Gerontol Int. 2013;13(2):393-397. [26] GIUSTI A, DE VINCENTIIS A, FRATONI F, et al. Effect of repeated application of low-intensity pulsed electromagnetic fields (PEMF) on gait speed in older adults with a history of falls. J Am Geriatr Soc. 2014;62(6):1185-1186. [27] BENER A, SALEH NM, BHUGRA D. Depressive symptoms and bone mineral density in menopause and postmenopausal women:A still increasing and neglected problem. J Family Med Prim Care. 2016;5(1): 143-149. [28] BAHOUQ H, SOULAYMANI A. Depression, Quality of Life, and Self-Esteem of Moroccan Postmenopausal Women with Osteoporosis before the Occurrence of Fractures. J Menopausal Med. 2020;26(2):121-129. [29] BASHAR M, AHMED K, UDDIN MS, et al. Depression and Quality of Life among Postmenopausal Women in Bangladesh: A Cross-sectional Study. J Menopausal Med. 2017;23(3):172-181. [30] POH PSP, SEELIGER C, UNGER M, et al. Osteogenic Effect and Cell Signaling Activation of Extremely Low-Frequency Pulsed Electromagnetic Fields in Adipose-Derived Mesenchymal Stromal Cells.Stem Cells Int. 2018;2018:5402853. [31] EHNERT S, VAN GRIENSVEN M, UNGER M, et al.Co-Culture with Human Osteoblasts and Exposure to Extremely Low Frequency Pulsed Electromagnetic Fields Improve Osteogenic Differentiation of Human Adipose-Derived Mesenchymal Stem Cells. Int J Mol Sci. 2018;19(4):994. [32] ONGARO A, PELLATI A, BAGHERI L, et al. Pulsed electromagnetic fields stimulate osteogenic differentiation in human bone marrow and adipose tissue derived mesenchymal stem cells. Bioelectromagnetics. 2014;35(6):426-436. [33] CARROLL SH, WIGNER NA, KULKARNI N, et al. A2B adenosine receptor promotes mesenchymal stem cell differentiation to osteoblasts and bone formation in vivo. J Biol Chem. 2012;287(19):15718-15727. [34] WU S, YU Q, LAI A, et al.Pulsed electromagnetic field induces Ca(2+)-dependent osteoblastogenesis in C3H10T1/2 mesenchymal cells through the Wnt-Ca(2+)/Wnt-beta-catenin signaling pathway. Biochem Biophys Res Commun. 2018;503(2):715-721. [35] CHEN CT, SHIH YR, KUO TK, et al.Coordinated changes of mitochondrial biogenesis and antioxidant enzymes during osteogenic differentiation of human mesenchymal stem cells. Stem Cells. 2008;26(4):960-968. [36] EHNERT S, FENTZ AK, SCHREINER A, et al.Extremely low frequency pulsed electromagnetic fields cause antioxidative defense mechanisms in human osteoblasts via induction of *O2(-) and H2O2. Sci Rep. 2017; 7(1):14544. [37] GAVALI S, GUPTA MK, DASWANI B, et al.Estrogen enhances human osteoblast survival and function via promotion of autophagy. Biochim Biophys Acta Mol Cell Res. 2019;1866(9):1498-1507. [38] QI M, ZHANG L, MA Y, et al. Autophagy Maintains the Function of Bone Marrow Mesenchymal Stem Cells to Prevent Estrogen Deficiency-Induced Osteoporosis. Theranostics. 2017;7(18):4498-4516. [39] FERRONI L, GARDIN C, DOLKART O, et al. Pulsed electromagnetic fields increase osteogenetic commitment of MSCs via the mTOR pathway in TNF-alpha mediated inflammatory conditions: an in-vitro study. Sci Rep. 2018;8(1):5108. [40] ADAMOPOULOS IE. Inflammation in bone physiology and pathology. Curr Opin Rheumatol. 2018;30(1):59-64. [41] ZHANG Y, ZHU X, WANG G, et al. Melatonin Rescues the Ti Particle-Impaired Osteogenic Potential of Bone Marrow Mesenchymal Stem Cells via the SIRT1/SOD2 Signaling Pathway. Calcif Tissue Int. 2020;107(5):474-488. [42] SONG J, LI J, YANG F, et al.Nicotinamide mononucleotide promotes osteogenesis and reduces adipogenesis by regulating mesenchymal stromal cells via the SIRT1 pathway in aged bone marrow. Cell Death Dis. 2019;10(5):336. [43] HE Z, SELVAMURUGAN N, WARSHAW J,et al.Pulsed electromagnetic fields inhibit human osteoclast formation and gene expression via osteoblasts. Bone. 2018;106(1):194-203. [44] DINIZ P, SHOMURA K, SOEJIMA K, et al. Effects of pulsed electromagnetic field (PEMF) stimulation on bone tissue like formation are dependent on the maturation stages of the osteoblasts.Bioelectromagnetics. 2002;23(5):398-405. [45] TEVEN CM, GREIVES M, NATALE RB, et al.Differentiation of osteoprogenitor cells is induced by high-frequency pulsed electromagnetic fields. J Craniofac Surg. 2012;23(2):586-593. [46] CHANG WH, CHEN LT, SUN JS, et al.Effect of pulse-burst electromagnetic field stimulation on osteoblast cell activities. Bioelectromagnetics. 2004;25(6):457-465. [47] SOLLAZZO V, PALMIERI A, PEZZETTI F, et al. Effects of pulsed electromagnetic fields on human osteoblastlike cells (MG-63): a pilot study. Clin Orthop Relat Res. 2010;468(8):2260-2277. [48] ZHOU J, WANG JQ, GE BF, et al. Different electromagnetic field waveforms have different effects on proliferation, differentiation and mineralization of osteoblasts in vitro. Bioelectromagnetics. 2014; 35(1):30-38. [49] CAI J, SHAO X, YAN Z, et al.Differential skeletal response in adult and aged rats to independent and combinatorial stimulation with pulsed electromagnetic fields and mechanical vibration. FASEB J. 2020;34(2): 3037-3050. [50] ZHOU J, LI X, LIAO Y, et al. Pulsed electromagnetic fields inhibit bone loss in streptozotocin-induced diabetic rats. Endocrine. 2015;49(1): 258-266. [51] MANCINI L, MORADI-BIDHENDI N, et al. The biphasic effects of nitric oxide in primary rat osteoblasts are cGMP dependent. Biochem Biophys Res Commun. 2000;274(2):477-481. [52] 任茜, 王鸣刚, 陈克明, 等.脉冲电磁场促进大鼠成骨细胞成熟与矿化依赖于NO/cGMP信号途径[J].中国生物化学与分子生物学报, 2016,32(11):1242-1248. [53] 王媛媛. 低频脉冲电磁场通过初级纤毛启动sAC/cAMP/PKA/CREB途径促进骨形成的研究[D].兰州:兰州理工大学,2018. [54] WANG YY, PU XY, SHI WG, et al. Pulsed electromagnetic fields promote bone formation by activating the sAC-cAMP-PKA-CREB signaling pathway. J Cell Physiol. 2019;234(3):2807-2821. [55] YIN C, TIAN Y, YU Y, et al. A novel long noncoding RNA AK016739 inhibits osteoblast differentiation and bone formation. J Cell Physiol. 2019;234(7):11524-11536. [56] YANG L, LI Y, GONG R, et al. The Long Non-coding RNA-ORLNC1 Regulates Bone Mass by Directing Mesenchymal Stem Cell Fate. Mol Ther. 2019;27(2):394-410. [57] WANG Y, WANG K, ZHANG L, et al. Targeted overexpression of the long noncoding RNA ODSM can regulate osteoblast function in vitro and in vivo.Cell Death Dis. 2020;11(2):133. [58] TSCHON M, VERONESI F, CONTARTESE D, et al. Effects of pulsed electromagnetic fields and platelet rich plasma in preventing osteoclastogenesis in an in vitro model of osteolysis. J Cell Physiol. 2018;233(3):2645-2656. [59] SONG ZH, XIE W, ZHU SY, et al. Effects of PEMFs on Osx, Ocn, TRAP, and CTSK gene expression in postmenopausal osteoporosis model mice. Int J Clin Exp Pathol. 2018;11(3):1784-1790. [60] NOH JY, YANG Y, JUNG H. Molecular Mechanisms and Emerging Therapeutics for Osteoporosis. Int J Mol Sci. 2020;21(20):7623. [61] 李忠浩, 丁宁, 杨全增, 等.破骨细胞中NFATc1相关调节研究进展[J].中国骨质疏松杂志,2017,23(5): 695-700. [62] ZHANG J, XU H, HAN Z, et al. Pulsed electromagnetic field inhibits RANKL-dependent osteoclastic differentiation in RAW264.7 cells through the Ca(2+)-calcineurin-NFATc1 signaling pathway. Biochem Biophys Res Commun. 2017;482(2):289-295. [63] HONG JM, KANG KS, YI HG, et al. Electromagnetically controllable osteoclast activity.Bone. 2014;62(3)99-107. [64] LEE SY, PARK KH, YU HG, et al. Controlling hypoxia-inducible factor-2alpha is critical for maintaining bone homeostasis in mice. Bone Res. 2019;7:14. [65] PRIDEAUX M, FINDLAY DM, ATKINS GJ. Osteocytes: The master cells in bone remodelling.Curr Opin Pharmacol. 2016;28:24-30. [66] WANG P, TANG C, WU J, et al. Pulsed electromagnetic fields regulate osteocyte apoptosis, RANKL/OPG expression, and its control of osteoclastogenesis depending on the presence of primary cilia. J Cell Physiol. 2019;234(7):10588-10601. |
| [1] | Wang Jing, Xiong Shan, Cao Jin, Feng Linwei, Wang Xin. Role and mechanism of interleukin-3 in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1260-1265. |
| [2] | Hou Jingying, Guo Tianzhu, Yu Menglei, Long Huibao, Wu Hao. Hypoxia preconditioning targets and downregulates miR-195 and promotes bone marrow mesenchymal stem cell survival and pro-angiogenic potential by activating MALAT1 [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1005-1011. |
| [3] | Liang Xuezhen, Yang Xi, Li Jiacheng, Luo Di, Xu Bo, Li Gang. Bushen Huoxue capsule regulates osteogenic and adipogenic differentiation of rat bone marrow mesenchymal stem cells via Hedgehog signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1020-1026. |
| [4] | Fang Xiaolei, Leng Jun, Zhang Chen, Liu Huimin, Guo Wen. Systematic evaluation of different therapeutic effects of mesenchymal stem cell transplantation in the treatment of ischemic stroke [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1085-1092. |
| [5] | Tian Chuan, Zhu Xiangqing, Yang Zailing, Yan Donghai, Li Ye, Wang Yanying, Yang Yukun, He Jie, Lü Guanke, Cai Xuemin, Shu Liping, He Zhixu, Pan Xinghua. Bone marrow mesenchymal stem cells regulate ovarian aging in macaques [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 985-991. |
| [6] | Zhang Jianguo, Chen Chen, Hu Fengling, Huang Daoyu, Song Liang. Design and biomechanical properties of dental implant pore structure based on three-dimensional finite element analysis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 585-590. |
| [7] | Kang Kunlong, Wang Xintao. Research hotspot of biological scaffold materials promoting osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 597-603. |
| [8] | Wu Zhongshu, Wei Yurou, Chen Xiaojun, Wuri Shana, He Wei, Wei Qiushi. Huo Xue Tong Luo capsule improves osteoblastogenesis of bone marrow mesenchymal stem cells through the ERα-Wnt/β-catenin signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(25): 3937-3943. |
| [9] | Ji Hangyu, Gu Jun, Xie Linghan, Bao Junping, Peng Xin, Wu Xiaotao. Chitosan/poly(lactic-co-glycolic acid)/polylactic acid scaffold with sustained release of nerve growth factor promotes the differentiation of bone marrow mesenchymal stem cells into neurons [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(25): 3974-3979. |
| [10] | Zeng Yuwei, Huang Chuang, Wei Jianguo, Duan Dongming, Wang Le. Tracing transplanted bone marrow mesenchymal stem cells in rat calvarial defect by bioluminescence imaging [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(25): 3968-3973. |
| [11] | Zhang Jingying, Li Ziyi, Liu Xiaochuan, Li Dan, Wang Yang, Wu zhuguo. Tail vein injection of bone marrow mesenchymal stem cells for repair of skull injury in aging mice [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(25): 3944-3950. |
| [12] | Chen Qiaoling, Bai Yiguang, Liu Kang, Lin Tao, Luo Xuwei. Osteoblast differentiation after conditional knockout of 3-phosphoinositide-dependent protein kinase-1 gene from bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(24): 3785-3789. |
| [13] | Chen Chichi, Zhang Yu, He Jiachen, Shi Qin. Osteogenic differentiation of bone marrow mesenchymal stem cells in obese mice [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(24): 3846-3851. |
| [14] | You Wulin, Huang Guicheng, Wang Jianwei. Osteogenic differentiation potential of microencapsulated transgenic bone marrow mesenchymal stem cells cocultured with osteoblasts [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(24): 3852-3857. |
| [15] | Cui Yinpeng, Guo Ai, Ma Lifeng, Liu Zhenjiang. Ouabain induces in vitro differentiation of human bone marrow mesenchymal stem cells into osteoblasts [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(24): 3796-3801. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||