Chinese Journal of Tissue Engineering Research ›› 2022, Vol. 26 ›› Issue (25): 3968-3973.doi: 10.12307/2022.400
Previous Articles Next Articles
Tracing transplanted bone marrow mesenchymal stem cells in rat calvarial defect by bioluminescence imaging
Zeng Yuwei, Huang Chuang, Wei Jianguo, Duan Dongming, Wang Le
- Department of Orthopedics, Third Affiliated Hospital of Guangzhou Medical University, Guangzhou 510150, Guangdong Province, China
-
Received:2021-03-11Accepted:2021-05-07Online:2022-09-08Published:2022-01-25 -
Contact:Wang Le, MD, Associate chief physician, Associate professor, Department of Orthopedics, Third Affiliated Hospital of Guangzhou Medical University, Guangzhou 510150, Guangdong Province, China -
About author:Zeng Yuwei, Master candidate, Department of Orthopedics, Third Affiliated Hospital of Guangzhou Medical University, Guangzhou 510150, Guangdong Province, China -
Supported by:the National Natural Science Foundation of China (General Program), No. 82072409 (to WL); Natural Science Foundation of Guangdong Province, No. 2019A1515012020 (to WL)
CLC Number:
Cite this article
Zeng Yuwei, Huang Chuang, Wei Jianguo, Duan Dongming, Wang Le. Tracing transplanted bone marrow mesenchymal stem cells in rat calvarial defect by bioluminescence imaging[J]. Chinese Journal of Tissue Engineering Research, 2022, 26(25): 3968-3973.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
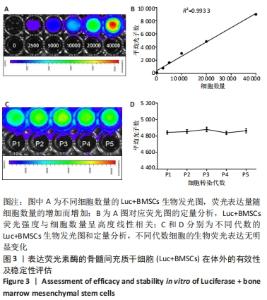
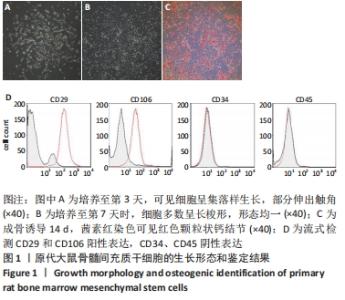
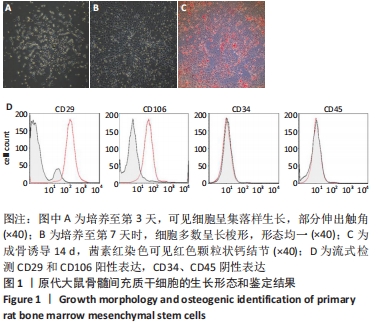
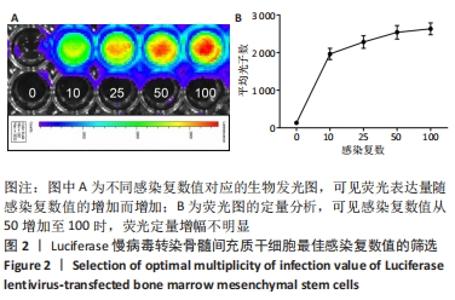
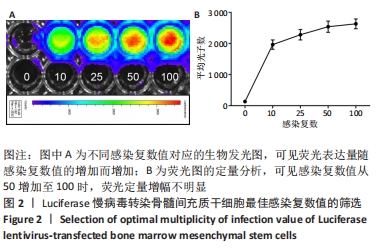
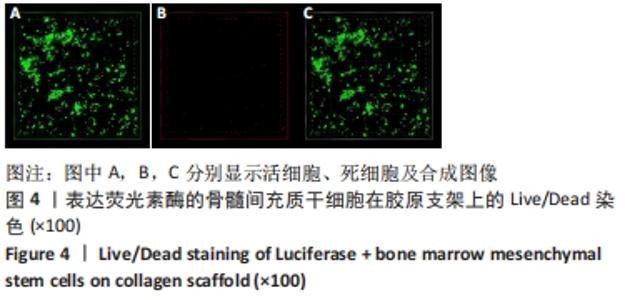
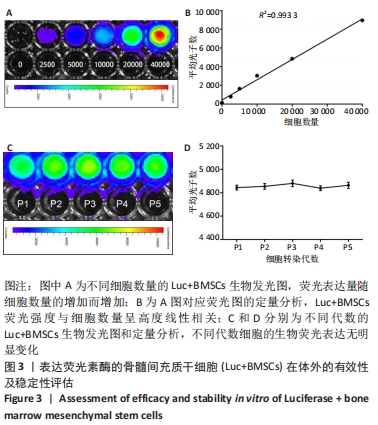
2.3 Luc+BMSCs在体外的有效性及稳定性评估 为了检验Luc+BMSCs作为示踪细胞的有效性,根据上述最佳感染复数获得大量稳转Luc+BMSCs,然后进行梯度细胞数量和生物荧光定量的相关性分析。从细胞生物荧光中可观察到生物荧光强度随细胞的数量增加而增加,见图3A。定量分析显示,在体外细胞水平,Luc+BMSCs荧光强度定量与细胞数量显示出同比例递增关系,呈现高度的线性相关(R2=0.993),见图3B,说明干细胞移植到动物体内后通过生物荧光定量值估计存活的细胞数量是有充分依据的。同时,为检验Luc+BMSCs在分裂增殖过程中生物荧光基因表达的稳定性,选取慢病毒转染后不同代数的细胞,观察Luc+BMSCs的荧光表达是否变化。不同代数的Luc+BMSCs均能稳定表达生物荧光,未表现出荧光强度随代数增加而衰减的现象,见图3C,荧光定量显示荧光值无明显差异,见图3D。"
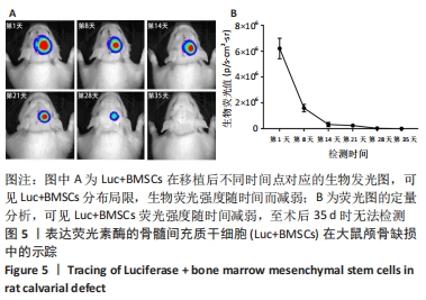
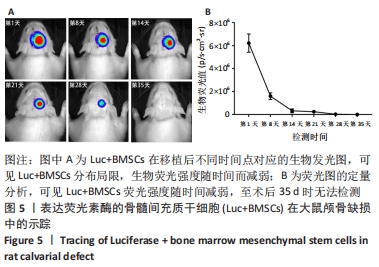
2.5 Luc+BMSCs在大鼠颅骨缺损中的活体示踪 5×105个Luc+BMSCs在颅骨缺损中随时间变化的分布、存活情况,见图5A。术后第1天时,移植细胞的生物发光信号集中在颅骨缺损中心,并呈同心圆状分布,提示缺损中心区细胞密度较高,而分布范围明显超出颅骨缺损大小,提示移植细胞向邻近的肌肉或皮肤迁移和扩散。 随时间进展,生物荧光信号仍然固定在缺损区,但分布范围逐渐缩小,荧光强度随之进一步减弱,说明移植的干细胞在局部逐渐消失,至术后第35天时荧光降至无法检测。以术后第1天的荧光定量值为基准,细胞移植后第1,2,3,4,5周的存活率分别为25.67%,5.22%,3.94%,0.47%,0.00%。细胞生物荧光消失速度显示出“先快后慢”的规律,见图5B。"
| [1] PITTENGER MF, DISCHER DE, PÉAULT BM, et al. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med. 2019;4:22. [2] WANG X, ZHAO Z, ZHANG H, et al. Simultaneous isolation of mesenchymal stem cells and endothelial progenitor cells derived from murine bone marrow. Exp Ther Med. 2018;16(6):5171-5177. [3] CHEN L, LIU G, LI W, et al. Chondrogenic differentiation of bone marrow-derived mesenchymal stem cells following transfection with Indian hedgehog and sonic hedgehog using a rotary cell culture system. Cell Mol Biol Lett. 2019;24:16. [4] ARTHUR A, GRONTHOS S. Clinical Application of Bone Marrow Mesenchymal Stem/Stromal Cells to Repair Skeletal Tissue. Int J Mol Sci. 2020;21(24):9759. [5] 刘志燕,龙瀛,何晓晓,等.干细胞移植后示踪技术的研究进展[J].组织工程与重建外科杂志,2019,15(2):111-114. [6] ZHOU B, ZHAO Z, ZHANG X, et al. Effect of Allogenic Bone Marrow Mesenchymal Stem Cell Transplantation on T Cells of Old Mice. Cell Reprogram. 2020;22(1):30-35. [7] XIANG Y, WANG W, GAO Y, et al. Production and Characterization of an Integrated Multi-Layer 3D Printed PLGA/GelMA Scaffold Aimed for Bile Duct Restoration and Detection. Front Bioeng Biotechnol. 2020;8:971. [8] JIANG Z, YU K, FENG Y, et al. An effective light activated TiO2 nanodot platform for gene delivery within cell sheets to enhance osseointegration. Chem Eng J. 2020;402(5):126170. [9] LI Y, LIU L, YU Z, et al. Effects of Edaravone on Functional Recovery of a Rat Model with Spinal Cord Injury Through Induced Differentiation of Bone Marrow Mesenchymal Stem Cells into Neuron-Like Cells. Cell Reprogram. 2021; 23(1):47-56. [10] PIÑERO G, USACH V, SOTO PA, et al. EGFP transgene: a useful tool to track transplanted bone marrow mononuclear cell contribution to peripheral remyelination. Transgenic Res. 2018;27(2):135-153. [11] OZAWA T, YOSHIMURA H, KIM SB. Advances in fluorescence and bioluminescence imaging. Anal Chem. 2013;85(2):590-609. [12] 韩杰,王敬梓,周锐,等.尾静脉注射荧光素酶标记的膀胱癌T24细胞动物转移模型的构建[J].中华实验外科杂志,2019,36(2):361-363. [13] ASWENDT M, VOGEL S, SCHÄFER C, et al. Quantitative in vivo dual-color bioluminescence imaging in the mouse brain. Neurophotonics. 2019;6(2):025006. [14] CONWAY M, XU T, KIRKPATRICK A, et al. Real-time tracking of stem cell viability, proliferation, and differentiation with autonomous bioluminescence imaging. BMC Biol. 2020;18(1):79. [15] MEZZANOTTE L, VAN ‘T ROOT M, KARATAS H, et al. In Vivo Molecular Bioluminescence Imaging: New Tools and Applications. Trends Biotechnol. 2017;35(7):640-652. [16] 赵年欢,崔邦平,王朋,等.生物发光成像示踪干细胞移植的应用进展[J].巴楚医学,2018,1(1):125-128. [17] WU H, KANG N, WANG Q, et al. The Dose-Effect Relationship Between the Seeding Quantity of Human Marrow Mesenchymal Stem Cells and In Vivo Tissue-Engineered Bone Yield. Cell Transplant. 2015;24(10):1957-1968. [18] 张雅雯,朱光旭,李雅喆,等.一种改良大鼠颅骨缺损动物模型的构建及应用[J].实验动物与比较医学,2020,40(3):227-231. [19] 肖渝,王坤,李彦林,等.三种携带GFP慢病毒转染免骨髓间充质干细胞的效果[J].中国组织工程研究,2017,29(21):4642-4647. [20] 李燕,陆伟,竺丽梅,等.慢病毒介导的GFP转染对小鼠骨髓间充质干细胞表型、增殖和分化能力的影响[J].南京大学学报(自然科学),2014,50(6): 883-889. [21] Lukashev AN, Zamyatnin AA Jr. Viral Vectors for Gene Therapy: Current State and Clinical Perspectives. Biochemistry (Mosc). 2016;81(7):700-708. [22] 闫锦玉,邢万红.慢病毒介导Notch1基因shRNA沉默大鼠骨髓间充质干细胞Notch信号通路的表达[J].中国组织工程研究,2019,23(17):2659-2664. [23] 徐宠俊,何荷蕃,林群,等.人肝细胞生长因子基因慢病毒载体的构建及其在骨髓间充质干细胞中的表达[J].吉林大学学报(医学版),2021,47(1):16-24. [24] 李琦,邹志余,杨瑞泽,等.慢病毒载体介导增强型绿色荧光蛋白筛选稳定转染的兔骨髓间充质干细胞[J].西安交通大学学报(医学版),2019,40(4): 619-623. [25] LU L, LIU Y, ZHANG X, et al. The therapeutic role of bone marrow stem cell local injection in rat experimental periodontitis. J Oral Rehabil. 2020;47 Suppl 1:73-82. [26] WANG WH, SHEN CY, CHIEN YC, et al. Validation of Enhancing Effects of Curcumin on Radiotherapy with F98/FGT Glioblastoma-Bearing Rat Model. Int J Mol Sci. 2020;21(12):4385. [27] ANSARI AM, AHMED AK, MATSANGOS AE, et al. Cellular GFP Toxicity and Immunogenicity: Potential Confounders in in Vivo Cell Tracking Experiments. Stem Cell Rev Rep. 2016;12(5):553-559. [28] BRESSER K, DIJKGRAAF FE, PRITCHARD CEJ, et al. A mouse model that is immunologically tolerant to reporter and modifier proteins. Commun Biol. 2020;3(1):273. [29] WU Y, CHEN L, SCOTT PG, et al. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25(10):2648-2659. [30] VILALTA M, DÉGANO IR, BAGÓ J, et al. Biodistribution, long-term survival, and safety of human adipose tissue-derived mesenchymal stem cells transplanted in nude mice by high sensitivity non-invasive bioluminescence imaging. Stem Cells Dev. 2008;17(5):993-1003. [31] KALLMEYER K, ANDRÉ-LÉVIGNE D, BAQUIÉ M, et al. Fate of systemically and locally administered adipose-derived mesenchymal stromal cells and their effect on wound healing. Stem Cells Transl Med. 2020;9(1):131-144. [32] LIU J, SHI Y, HAN J, et al. Quantitative Tracking Tumor Suppression Efficiency of Human Umbilical Cord-Derived Mesenchymal Stem Cells by Bioluminescence Imaging in Mice Hepatoma Model. Int J Stem Cells. 2020;13(1):104-115. [33] LEE J, LEE S, AHMAD T, et al. Human adipose-derived stem cell spheroids incorporating platelet-derived growth factor (PDGF) and bio-minerals for vascularized bone tissue engineering. Biomaterials. 2020; 255:120192. [34] ROUX BM, VAICIK MK, SHRESTHA B, et al. Induced Pluripotent Stem Cell-Derived Endothelial Networks Accelerate Vascularization But Not Bone Regeneration. Tissue Eng Part A. 2020 Oct 19. doi: 10.1089/ten.TEA.2020.0200. [35] TEE BC, SUN Z. Xenogeneic mesenchymal stem cell transplantation for mandibular defect regeneration. Xenotransplantation. 2020;27(5):e12625. [36] ALVARADO-VELEZ M, ENAM SF, MEHTA N, et al. Immuno-suppressive hydrogels enhance allogeneic MSC survival after transplantation in the injured brain. Biomaterials. 2021;266:120419. [37] CHIARUGI P, GIANNONI E. Anoikis: a necessary death program for anchorage-dependent cells. Biochem Pharmacol. 2008;76(11):1352-1364. [38] GARCÍA-SÁNCHEZ D, FERNÁNDEZ D, RODRÍGUEZ-REY JC, et al. Enhancing survival, engraftment, and osteogenic potential of mesenchymal stem cells. World J Stem Cells. 2019;11(10):748-763. [39] MEHRBANI AZAR Y, NIESLER CU, VAN DE VYVER M. Ex vivo antioxidant preconditioning improves the survival rate of bone marrow stem cells in the presence of wound fluid. Wound Repair Regen. 2020;28(4):506-516. [40] GOODMAN SB, LIN T. Modifying MSC Phenotype to Facilitate Bone Healing: Biological Approaches. Front Bioeng Biotechnol. 2020;8:641. |
| [1] | Wang Jianping, Zhang Xiaohui, Yu Jinwei, Wei Shaoliang, Zhang Xinmin, Xu Xingxin, Qu Haijun. Application of knee joint motion analysis in machanism based on three-dimensional image registration and coordinate transformation [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(在线): 1-5. |
| [2] | Li Huo, Wang Peng, Gao Jianming, Jiang Haoran, Lu Xiaobo, Peng Jiang. Relationship between revascularization and internal microstructure changes in osteonecrosis of the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1323-1328. |
| [3] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [4] | Bao Xianguo, Gao Zengxin, Wu Zhanpo, Chen Youmin, Cheng Qinghua, Lu Haitao, Guo Changzheng, Xu Shuai. Correlation between lumbar posterior muscle and local kyphosis in patients with degenerative thoracolumbar kyphosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1418-1423. |
| [5] | Zhang Haobo, Zhao Yunan, Yang Xuejun. Role and therapeutic implications of pyroptosis in intervertebral disc degeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1445-1451. |
| [6] | Jing Jinpeng, Zhang Yue, Liu Xiaomin, Liu Yi. Traditional Chinese medicine injection for promoting blood circulation in prevention of deep vein thrombosis after orthopedic surgery: network meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1467-1476. |
| [7] | Gu Zhengqiu, Xu Fei, Wei Jia, Zou Yongdi, Wang Xiaolu, Li Yongming. Exploratory study on talk test as a measure of intensity in blood flow restriction training [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1154-1159. |
| [8] | Kong Yamin, Yan Juntao, Ma Bingxiang, Li Huawei. Massage vibration intervenes with MyoD expression and proliferation and differentiation of muscle satellite cells in rats with sciatic nerve injury [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1160-1166. |
| [9] | Wu Cong, Jia Quanzhong, Liu Lun. Relationship between transforming growth factor beta1 expression and chondrocyte migration in adult articular cartilage after fragmentation [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1167-1172. |
| [10] | Li Zhiyi, He Pengcheng, Bian Tianyue, Xiao Yuxia, Gao Lu, Liu Huasheng. Bibliometric and visualized analysis of ferroptosis mechanism research [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1202-1209. |
| [11] | Xiang Xinjian, Liu Fang, Wu Liangliang, Jia Daping, Tao Yue, Zhao Zhengnan, Zhao Yu. High-dose vitamin C promotes the survival of autologous fat transplantation in rats [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1242-1246. |
| [12] | Wang Jing, Xiong Shan, Cao Jin, Feng Linwei, Wang Xin. Role and mechanism of interleukin-3 in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1260-1265. |
| [13] | Xiao Hao, Liu Jing, Zhou Jun. Research progress of pulsed electromagnetic field in the treatment of postmenopausal osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1266-1271. |
| [14] | Zhu Chan, Han Xuke, Yao Chengjiao, Zhang Qiang, Liu Jing, Shao Ming. Acupuncture for Parkinson’s disease: an insight into the action mechanism in animal experiments [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1272-1277. |
| [15] | Tian Chuan, Zhu Xiangqing, Yang Zailing, Yan Donghai, Li Ye, Wang Yanying, Yang Yukun, He Jie, Lü Guanke, Cai Xuemin, Shu Liping, He Zhixu, Pan Xinghua. Bone marrow mesenchymal stem cells regulate ovarian aging in macaques [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 985-991. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||