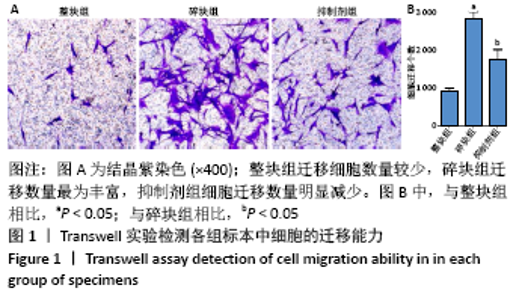
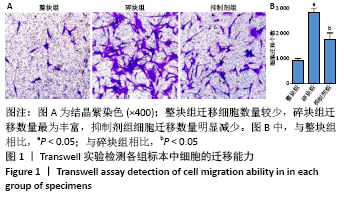
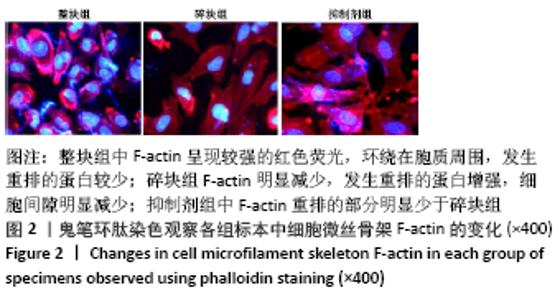
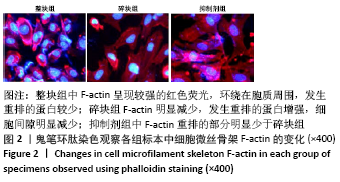
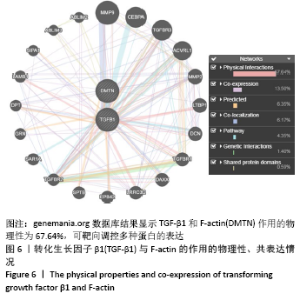
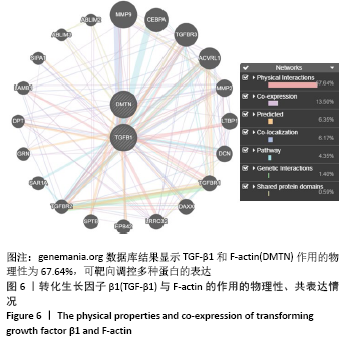
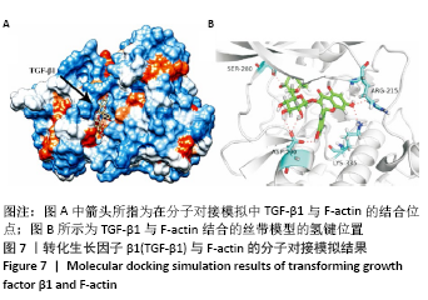
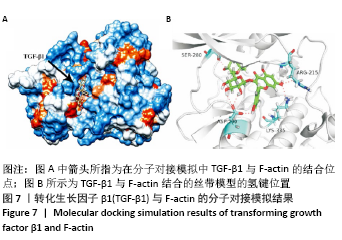
[1] BOWLAND P, COWIE RM, INGHAM E, et al. Biomechanical assessment of the stability of osteochondral grafts implanted in porcine and bovine femoral condyles. Proc Inst Mech Eng H. 2020;234(2):163-170.
[2] BEYNNON BD, FIORENTINO N, GARDNER-MORSE M, et al. Combined Injury to the ACL and Lateral Meniscus Alters the Geometry of Articular Cartilage and Meniscus Soon After Initial Trauma. J Orthop Res. 2020; 38(4):759-767.
[3] ZHENG Z, XIANG S, WANG Y, et al. NR4A1 promotes TNF α induced chondrocyte death and migration injury via activating the AMPK/Drp1/mitochondrial fission pathway. Int J Mol Med. 2020;45(1):151-161.
[4] YAUSEP OE, MADHI I, TRIGKILIDAS D. Platelet rich plasma for treatment of osteochondral lesions of the talus: A systematic review of clinical trials. J Orthop. 2020;18(1):218-225.
[5] LI S, NIU G, WU Y, et al. Vitamin D prevents articular cartilage erosion by regulating collagen II turnover through TGF-β1 in ovariectomized rats. Osteoarthritis Cartilage. 2016;24(2):345-353.
[6] WANG YJ, SHEN M, WANG S, et al. Inhibition of the TGF-β1/Smad signaling pathway protects against cartilage injury and osteoarthritis in a rat model. Life Sci. 2017;189(7):106-113.
[7] WANG Q, ZHOU C, LI X, et al. TGF-β1 promotes gap junctions formation in chondrocytes via Smad3/Smad4 signalling. Cell Prolif. 2019;52(2): e12544.
[8] CALKINS TE, CULVERN C, NAM D, et al. Dilute Betadine Lavage Reduces the Risk of Acute Postoperative Periprosthetic Joint Infection in Aseptic Revision Total Knee and Hip Arthroplasty: A Randomized Controlled Trial. J Arthroplasty. 2020;35(2):538-543.
[9] IKOMA K, KIDO M, MAKI M, et al. Early stage and small medial osteochondral lesions of the talus in the presence of chronic lateral ankle instability: A retrospective study. J Orthop Sci. 2020;25(1): 178-182.
[10] PARRENO J, KANDEL R, FOWLER V. Regionally distinct f-actin networks within articular cartilage. Osteoarthritis Cartilage. 2017;25(3):149-150.
[11] KIM IG, KO J, LEE HR, et al. Mesenchymal cells condensation-inducible mesh scaffolds for cartilage tissue engineering. Biomaterials. 2016;85(12):18-29.
[12] ROWLAND CR, COLUCCI LA, GUILAK F. Fabrication of anatomically-shaped cartilage constructs using decellularized cartilage-derived matrix scaffolds. Biomaterials. 2016;91(7):57-72.
[13] 宋登新, 曹云, 何小文, 等. 退行性骨关节炎软骨细胞差异表达蛋白及功能的研究[J]. 中华实验外科杂志,2015,32(8):1953-1956.
[14] LI G, SONG X, LI R, et al. Zyxin-involved actin regulation is essential in the maintenance of vinculin focal adhesion and chondrocyte differentiation status. Cell Prolif. 2019;52(1):e12532.
[15] CHINZEI N, BROPHY RH, DUAN X, et al. Molecular influence of anterior cruciate ligament tear remnants on chondrocytes: a biologic connection between injury and osteoarthritis. Osteoarthritis Cartilage. 2018;26(4): 588-599.
[16] ROTHDIENER M, UYNUK-ool t, sÜpkamp n, et al. Human osteoarthritic chondrons outnumber patient-and joint-matched chondrocytes in hydrogel culture-Future application in autologous cell-based OA cartilage repair? J Tissue Eng Regen Med. 2018;12(2): e1206-e1220.
[17] WANG Q, ZHOU C, LI X, et al. TGF-β1 promotes gap junctions formation in chondrocytes via Smad3/Smad4 signalling. Cell Prolif. 2019;52(2): e12544.
[18] HORITA M, NISHIDA K, HASEI J, et al. Involvement of ADAM12 in Chondrocyte Differentiation by Regulation of TGF-β1-Induced IGF-1 and RUNX-2 Expressions. Calcif Tissue Int. 2019;105(1):97-106.
[19] CHEN Y, WU T, HUANG S, et al. Sustained release SDF-1α/TGF-β1-loaded silk fibroin-porous gelatin scaffold promotes cartilage repair. ACS Appl Mater Interfaces. 2019;11(16):14608-14618.
|