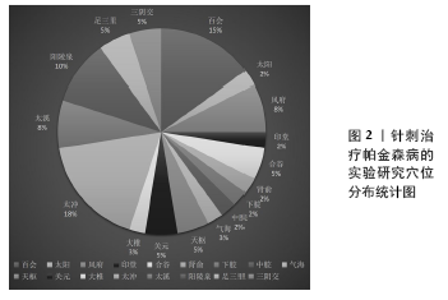
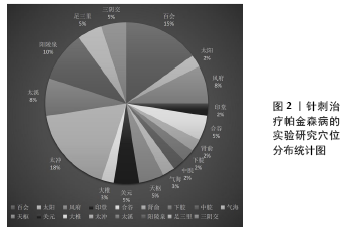
Chinese Journal of Tissue Engineering Research ›› 2022, Vol. 26 ›› Issue (8): 1272-1277.doi: 10.12307/2022.235
Previous Articles Next Articles
Acupuncture for Parkinson’s disease: an insight into the action mechanism in animal experiments
Zhu Chan1, 2, Han Xuke1, 2, Yao Chengjiao1, 2, Zhang Qiang3, Liu Jing4, Shao Ming4, 5
- 1Affiliated Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu 610072, Sichuan Province, China; 2Chengdu University of Traditional Chinese Medicine, Chengdu 610075, Sichuan Province, China; 3Sichuan Provincial Orthopedics Hospital, Chengdu 610041, Sichuan Province, China; 4Sichuan China 81 Rehabilitation Center (Sichuan Provincial Rehabilitation Hospital), Chengdu 611135, Sichuan Province, China; 5Brain Hospital Affiliated to Guangzhou Medical University, Guangzhou 510370, Guangdong Province, China
-
Received:2020-12-08Revised:2020-12-12Accepted:2021-01-09Online:2022-03-18Published:2021-11-02 -
Contact:Shao Ming, Chief physician, Doctoral supervisor, Sichuan China 81 Rehabilitation Center (Sichuan Provincial Rehabilitation Hospital), Chengdu 611135, Sichuan Province, China; Brain Hospital Affiliated to Guangzhou Medical University, Guangzhou 510370, Guangdong Province, China -
About author:Zhu Chan, MD candidate, Attending physician, Affiliated Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu 610072, Sichuan Province, China; Chengdu University of Traditional Chinese Medicine, Chengdu 610075, Sichuan Province, China -
Supported by:the National Key Research and Development Program of China, No. 2018YFC2001600; Guangdong Provincial Key Research and Development Project, No. 2018B030339001; Sichuan Provincial Science and Technology Project, No. 2018JY0664 (to SM); China Rehabilitation Medical Institution Alliance Special Major Project, No. 20160001 (to SM); Research project of Sichuan Medical Association, No. S16061 (to SM)
CLC Number:
Cite this article
Zhu Chan, Han Xuke, Yao Chengjiao, Zhang Qiang, Liu Jing, Shao Ming. Acupuncture for Parkinson’s disease: an insight into the action mechanism in animal experiments[J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1272-1277.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
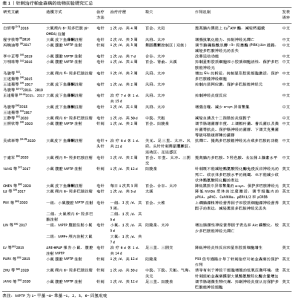
2.2 针刺干预帕金森病作用机制 针刺干预帕金森病的作用机制主要体现在增加脑内神经营养因子、减少脑内异常代谢产物、增强自噬、减少α-突触核蛋白(α-synuclein,α-syn)聚集、抑制神经细胞凋亡、抑制氧化应激、抑制内质网应激、调节肠道菌群及减轻神经炎症反应等方面,结果见表1。 2.2.1 针刺可以增加脑内神经营养因子 神经营养因子是重要的调节蛋白,能促进不同神经系统细胞的存活[36-37]。尤其是脑源性神经营养因子和胶质细胞源神经营养因子都能影响帕金森病患者。而Pitx3是黑质纹状体多巴胺能神经元中胶质细胞源神经营养因子诱导脑源性神经营养因子表达的关键递质[37-39]。电针百会、大椎能上调黑质多巴胺能神经元cAMP反应元件结合蛋白(CREB)及Akt和Pitx3的表达,增加脑源性神经营养因子和胶质细胞源神经营养因子的表达,减轻帕金森病小鼠运动功能障碍和多巴胺能神经元变性[30]。电针阳陵泉、太冲可以增加脑源性神经营养因子表达和Akt磷酸化减少MPP+诱导的大鼠黑质多巴胺能神经元凋亡[31]。 2.2.2 针刺减轻黑质纹状体谷氨酸过量 谷氨酸转运系统功能异常与帕金森病关系密切。过量的谷氨酸导致帕金森病黑质纹状体神经元丢失,谷氨酸转运体1表达减少影响谷氨酸再摄取,引起神经细胞的兴奋性毒性。中枢神经系统谷氨酸生理浓度的维持主要依赖神经细胞和神经胶质细胞上谷氨酸转运体1的参与,该转运体功能或结构改变直接造成谷氨酸堆积或升高,从而引起神经细胞毒作用,导致多巴胺能神经元的死亡,引起帕金森病[40]。有研究发现高频电刺激偏侧帕金森病猴模型的丘脑底核后,同侧的苍白球内侧部细胞外液中谷氨酸的含量明显上升[41];6-羟多巴胺制备的帕金森病大鼠模型组谷氨酸水平从建模后第3-6周逐渐升高,而多巴胺含量逐渐下降[12-13];这些研究均表明,谷氨酸浓度的升高与帕金森病的发生有直接而密切的关系。针刺风府、太冲能提高脑内谷氨酸转运体1 mRNA与谷氨酰胺合成酶(GSmRNA)、谷氨酰胺酶(PAG)蛋白活性表达,减轻谷氨酸引起的细胞毒作用,从而对多巴胺能神经元起到一定的保护作用。 2.2.3 针刺减轻细胞内钙超载 钙超载的病理现象参与帕金森病多巴胺能神经元损伤的发生发展,钙负荷在细胞内加重,能够促进氧化应激、加重线粒体损伤等致病因素,进而又加深钙超载程度,使神经元不断损伤,最终能量耗竭而死亡[42]。沿皮百会透刺太阳可以提高大鼠脑内黑质上Ca2+-ATP酶,调节大鼠脑内黑质的Calbindin-D28k蛋白表达,从而抑制帕金森病大鼠脑内黑质Ca2+浓度的升高,保护多巴胺能神经细胞。 2.2.4 针刺抑制多巴胺能神经细胞凋亡 神经细胞凋亡是不同因素引起帕金森病发病过程中的一条共同的通道,bcl-2在发育过程的大脑中广泛表达,是细胞死亡(氧化应激、脂质过氧化和线粒体呼吸受损等)的重要调节因子,在抗凋亡中存在积极影响;相反,bax作为各种神经细胞损伤的启动子,在诱导和启动黑质细胞凋亡方面起关键作用[43]。电针关元、足三里、太冲、风府,头针针刺舞蹈震颤区、运动区、足运感区可以提高帕金森病大鼠黑质多巴胺能神经元合成多巴胺功能,改善大鼠行为学,上调凋亡相关蛋白Bcl-2表达,抑制Bax表达[24-25]。普通针刺阳陵泉、足三里治疗1-甲基-4-苯基-1,2,3,6-四氢吡啶(1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine,MPTP)诱导的帕金森病模型小鼠也出现bcl-2水平下降,bax水平增高[35]。 2.2.5 针刺抗氧化应激 氧化损伤和线粒体功能障碍是帕金森病神经元死亡主要原因。抗氧化转录因子-核因子E2相关因子2 (nuclear factor-E2-related factor-2,Nrf2)在决定抗氧化能力方面起主要作用,它参与氧化还原动态平衡,调节一系列抗氧化酶。电针风府、太冲通过提高抗超氧阴离子氧活性,增强抗氧化能力途径抑制神经元细胞凋亡[8],电针足三里、三阴交可以逆转MPTP造成的纹状体、中脑中Nrf2以及Nrf2调节的烟酰胺腺嘌呤二核苷酸磷酸醌氧化还原酶1和血红素加氧酶的降低[32]。 2.2.6 针刺抗内质网应激 内质网是蛋白质合成、修饰与加工、折叠、组装和运输的场所,其功能易受到各种刺激因素干扰导致未折叠或错误折叠蛋白在腔内蓄积,进而发生内质网应激[44]。当发生内质网应激时,细胞激活未折叠蛋白反应来应对错误蛋白的折叠与积累。定位于内质网膜上的活化转录因子ATF6能够感受内质网膜未折叠蛋白聚集的信号[45],通过调节相关基因的表达来缓解内质网压力[46];CHOP活化途径作为内质网应激介导的细胞凋亡途径之一,是内质网应激下游信号传导通路一个关键的特异性转录因子,CHOP诱导抗凋亡蛋白Bcl-2表达下调诱发神经细胞凋亡[47]。内质网分子伴侣免疫球蛋白重链结合蛋白(Bip)是内质网应激的经典标志分子,在辅助蛋白质加工合成、调节内质网应激信号中扮演着重要角色,它可以帮助未折叠蛋白反应正确折叠,减轻内质网负荷,恢复内质网稳态,电针风府、太冲,可以抑制内质网相关应激基因的表达,下调应激相关因子的激活而保护多巴胺能神经元[14-17,48]。 2.2.7 针刺可以增强自噬,减少异常α-syn的聚集 α-syn的异常聚集产生具有神经毒性的寡聚体,最终导致多巴胺能神经元变性坏死。自噬-溶酶体途径是机体清除错误折叠蛋白异常沉积、聚集的有效方法[49],能在营养缺乏情况下为细胞生长代谢提供必要的大分子物质和能量并清除细胞内过剩或有缺陷的细胞器,是维持α-syn在多巴胺神经元蛋白稳态的主要途径之一[50],抑制自噬能加剧各型α-syn积聚而致使细胞死亡;上调自噬可以促进α-syn的降解以及细胞生长[51]。热休克同源蛋白7参与分子伴侣介导的自噬途径。溶酶体相关膜蛋白2A(Lysosome-associated membrane protein type-2a,Lamp2a)是分子伴侣介导的自噬的关键分子,在持续的缺乏营养、氧化应激以及毒物刺激等的情况下,Lamp2a的表达水平可见明显升高,并且共存溶酶体数量增多,集中分布在细胞核周围[52],通过对两者的检测,可评价分子伴侣介导的自噬活性。微管相关膜蛋白轻链3(microtubule associated protein light chain 3,LC3-Ⅱ)是自噬的关键性蛋白,能稳定地结合到自噬体膜上并一直存在于自噬体的各个阶段;P62也称SQSTM1,作为一种自噬底物蛋白,P62的蛋白含量可以反映自噬流的完成情况,在自噬进程中,P62蛋白通过直接结合LC3而选择性地被包裹进自噬体中被有效地降解。因此,细胞内P62总蛋白水平与自噬流水平负相关[53-54]。电针风府、太冲可以通过上调Lamp2A、热休克同源蛋白70、LC3-Ⅱ水平,抑制错误折叠蛋白α-syn的表达,降低自噬底物蛋白P62[20-21]。 2.2.8 针刺抑制神经炎症反应 帕金森病患者脑脊液和黑质纹状体系统炎性细胞因子均较健康对照组增高,包括白细胞介素1、肿瘤坏死因子α和干扰素γ等,这些细胞因子主要存在于黑质纹状体系统,而非大脑皮质区[55]。帕金森病模型组中脑黑质p-c-Jun表达水平明显升高,p-c-Jun表达于黑质区细胞的胞核内且其阳性细胞分布与酪氨酸羟化酶阳性细胞相一致,经JNK通路特异性抑制剂SP600125抑制p-c-Jun表达,中脑黑质p-c-Jun表达水平明显降低,酪氨酸羟化酶阳性细胞丢失现象明显减轻。电针风府、太冲通过MAPK/JNK信号通路抑制炎性反应早期大鼠黑质区ERK1/2的磷酸化,p-c-Jun、肿瘤坏死因子α、干扰素γ、白细胞介素1β蛋白表达明显减少,对帕金森病的发生发展起到一定的调节作用[18-19]。 2.2.9 针刺抑制胶质细胞的异常激活 胶质细胞对神经元起着支持、保护、信息传递等作用,在病理情况下,胶质细胞增殖合成并分泌多种免疫和炎症递质,参与抗原提呈[56]。但帕金森病状态下胶质细胞的过度激活,会进一步加速帕金森病的病程[57]。胶质纤维酸性蛋白是星形胶质细胞的特异性标志物,OX-42是小胶质细胞的特异性标志物[58]。采用“肾脑相济”法电针肾俞、百会、太溪可以抑制星形胶质细胞和小胶质细胞的活性,从而增加其对多巴胺能神经元的保护作用[11]。电针风府、太冲能够抑制星形胶质细胞激活,减少胶质纤维酸性蛋白 mRNA表达和谷氨酸释放[59]。阳陵泉、足三里行普通针刺也可以抑制胶质纤维酸性蛋白在黑质中的表达[35]。 2.2.10 针刺可以调节肠道菌群 研究发现,α-syn的异常折叠和聚集可能起源于肠神经系统,与肠道菌群存在密切联 系[60]。将帕金森病患者的肠道微生物移植到无菌小鼠后,可出现α-syn聚集、诱发神经炎症和运动障碍[61]。最新证据表明α-syn可能通过迷走神经从肠道扩散到大脑,迷走神经切断术和α-syn缺乏症阻止了α-syn病的肠脑扩散以及相关的神经变性和行为缺陷[62]。电针天枢、中脘可以降低血清中的白细胞介素1β和十二指肠中的肿瘤坏死因子α[22]。针刺足三里、阳陵泉可以使18个菌属的相对丰度发生变化[35],其中包括了与焦虑和运动功能障碍明显有关的菌属。针刺百会、阳陵泉可以显著调节肠道菌群丰度,并改善模型小鼠肠道微生物的多样性,上调拟杆菌、普氏菌以及粪杆菌等抗炎、保护肠神经的菌群,下调艾克曼菌等破坏肠道屏障的菌群[23]。 "
| [1] 陈生弟.中国帕金森病治疗指南(第三版)[J].中华神经科杂志,2014, 47(6):428-433. [2] 张森,赵晓悦,梁宇,等.帕金森病致病因素及发病机制研究进展[J].药学学报,2020,55(10):2264-2272. [3] NOH H, KWON S, CHO S, et al. Effectiveness and safety of acupuncture in the treatment of Parkinson’s disease: A systematic review and meta-analysis of randomized controlled trials. Complement Ther Med. 2017;34:86-103. [4] 朱文昕,奚桂芳,睢久红.针药治疗对帕金森病小鼠脑内多巴胺影响[J].针刺研究,1996(4):46-49. [5] 白妍,何便鸿,王东升,等.不同电针头穴透刺对PD大鼠不同时间点脑内黑质Ca~(2+)浓度的影响[J].中国中医药科技, 2016,23(2):180-181+236. [6] 白妍,胡丙成,阿木拉,等.不同电针头穴透刺对PD大鼠脑内黑质Calbindin-D28k蛋白表达的影响[J].中国中医药科技, 2016,23(2):178-180. [7] 白妍,卢金荣,胡丙成,等.不同电针头穴透刺对帕金森病大鼠脑内黑质钙稳态相关3种ATP酶活性的影响[J].中国中医药科技, 2016,23(1):4-5. [8] 程宇核,张少武,朱小虎,等.电针对帕金森病模型大鼠黑质超氧阴离子、超氧化物岐化酶的影响[J].临床和实验医学杂志,2016,15(21): 2073-2076. [9] 冯琬迪,王媛媛,盖聪,等.针刺联合美多芭对帕金森病小鼠脑多巴胺神经元及蛋白激酶B表达的影响[J].北京中医药大学学报,2017,40(3): 241-246. [10] 李中正,盛益华,李思迪,等.电针对MPTP亚急性帕金森病模型小鼠步态运动行为的影响[J].湖南中医药大学学报,2019,39(7):874-878. [11] 万明珠,任路,于嵩,等.“肾脑相济”电针疗法对帕金森病模型小鼠中脑黑质胶质细胞的影响[J].中医杂志,2018,59(18):1597-1601. [12] 马骏,刘芳,王述菊,等.电针对帕金森病模型大鼠纹状体Glu浓度、GLT-1mRNA和GSmRNA表达的影响[J]. 时珍国医国药,2015,26(12): 3050-3053. [13] 王述菊,马骏,刘芳,等.电针对帕金森病模型大鼠纹状体Glu浓度、GS和PAG表达的影响[J].世界科学技术-中医药现代化,2015,17(10): 2079-2082. [14] 王述菊,王中明,马骏,等.电针对帕金森病模型大鼠黑质内质网应激相关基因表达的影响[J].时珍国医国药,2017,28(6):1497-1500. [15] 马骏,王彬,王述菊,等.电针“风府、太冲”穴对帕金森病模型大鼠内质网应激相关蛋白表达的影响[J].中国康复医学杂志,2019,34(7): 772-777. [16] 马骏,王中明,王述菊,等.电针对帕金森病模型大鼠黑质内Bip、CHOP蛋白表达的影响[J].上海针灸杂志,2018,37(1):86-91. [17] 马骏,袁利,王述菊,等.电针对帕金森病大鼠中脑黑质转录活化因子6和转录因子X盒结合蛋白1 mRNA表达的影响[J].针刺研究,2019, 44(11):805-809. [18] 王述菊,马骏,王彦春,等.电针对鱼藤酮诱导的帕金森病模型大鼠黑质内ERK1/2及TNF-α的影响[J].中国老年学杂志,2015,35(20):5694-5697. [19] 王述菊,马骏,王彦春,等.电针对帕金森病模型大鼠黑质区c-Jun氨基末端激酶和TNF-α、IFN-γ、IL-1β蛋白表达的影响[J].中华中医药学刊, 2017,35(1):43-46. [20] 马骏,余沛豪,王述菊,等.电针对帕金森病模型大鼠脑黑质内Lamp2a、 Hsc70、α-syn表达的影响[J].中华中医药学刊,2018,36(4):859-862. [21] 王述菊,余沛豪,马骏,等.电针对鱼藤酮诱导的帕金森病模型大鼠黑质内自噬相关蛋白表达的影响[J].辽宁中医杂志,2017,44(9):1812-1815. [22] 王静,王安龙,范小明,等.经颅超声对电针治疗帕金森病模型大鼠的效果评价[J].中华医学超声杂志(电子版),2020,17(1):70-75. [23] 王照钦,钟蕊,高崚,等.针刺对帕金森病模型小鼠肠道菌群多样性的调节作用[J].中华中医药杂志,2020,35(5):2265-2270. [24] 吴成举,英锡相,陈靖,等.针刺疗法对帕金森病大鼠黑质神经元凋亡蛋白bcl-2、bax的影响[J].陕西中医,2020,41(1):8-11+133. [25] 吴成举,英锡相,马贤德,等.不同方法针灸对帕金森模型大鼠脑细胞神经元代谢影响[J].辽宁中医药大学学报,2020,22(6):12-15. [26] 于建军.电针对帕金森抑郁模型大鼠脑内单胺类神经递质含量影响研究[J].针灸临床杂志,2020,36(2):70-73. [27] YANG HJ, GAO Y, YUN JY, et al. Acupuncture does not protect against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced damage of dopaminergic neurons in a preclinical mouse model of Parkinson’s disease. Neuroreport. 2017;28(1):50-55. [28] CHEN LD, CHEN K, AI L,et al. Effect of electroacupuncture on dopaminergic neurons in a rat model of Parkinson’s disease based on the alpha-synuclein pathway. Mater Express. 2020;10(1):62-69. [29] LU KW, YANG J, HSIEH CL, et al. Electroacupuncture restores spatial learning and downregulates phosphorylated N-methyl-D-aspartate receptors in a mouse model of Parkinson’s disease. Acupunct Med. 2017;35(2):133-141. [30] PAK ME, AHN SM, JUNG DH, et al. Electroacupuncture Therapy Ameliorates Motor Dysfunction via Brain-Derived Neurotrophic Factor and Glial Cell Line-Derived Neurotrophic Factor in a Mouse Model of Parkinson’s Disease. J Gerontol A Biol Sci Med Sci. 2020;75(4):712-721. [31] LIN JG, CHEN CJ, YANG HB, et al. Electroacupuncture Promotes Recovery of Motor Function and Reduces Dopaminergic Neuron Degeneration in Rodent Models of Parkinson’s Disease. Int J Mol Sci. 2017;18(9):1846. [32] LV E, DENG J, YU Y, et al. Nrf2-ARE signals mediated the anti-oxidative action of electroacupuncture in an MPTP mouse model of Parkinson’s disease.Free Radic Res. 2015;49(11):1296-307. [33] PARK JY, CHOI H, BAEK S, et al. p53 signalling mediates acupuncture-induced neuroprotection in Parkinson’s disease.Biochem Biophys Res Commun. 2015;460(3):772-779. [34] ZHU LS, WANG J, LU J, et al. Effect of hemodynamic characteristic changes of the carotid artery on 6-OHDA-induced Parkinson’s disease model rats treated by Gut-acupuncture.Journal of King Saud University - Science. 2020; 32(6):2675-2681. [35] JANG JH, YEOM MJ, AHN S, et al. Acupuncture inhibits neuroinflammation and gut microbial dysbiosis in a mouse model of Parkinson’s disease. Brain Behav Immun. 2020;89:641-655. [36] Aron L,Klein R.Repairing the parkinsonian brain with neurotrophic factors.Trends Neurosci. 2011;34(2):88-100. [37] NASROLAHI A, MAHMOUDI J, AKBARZADEH A, et al. Neurotrophic factors hold promise for the future of Parkinson’s disease treatment: is there a light at the end of the tunnel? Rev Neurosci. 2018;29(5):475-489. [38] GILL SS, PATEL NK, HOTTON GR, et al.Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat Med. 2003;9(5):589-595. [39] PENG C, ARON L, KLEIN R, et al. Pitx3 is a critical mediator of GDNF-induced BDNF expression in nigrostriatal dopaminergic neurons.J Neurosci. 2011;31(36):12802-12815. [40] SALVATORE MF, DAVIS RW, ARNOLD JC, et al.Transient striatal GLT-1 blockade increases EAAC1 expression, glutamate reuptake, and decreases tyrosine hydroxylase phosphorylation at ser(19). Exp Neurol. 2012;234(2):428-436. [41] 李敬军,马羽,胡文瀚,等.丘脑底核高频电刺激对偏侧帕金森猴苍白球内侧部中氨基酸神经递质含量的影响[J].立体定向和功能性神经外科杂志,2012,25(2):68-71. [42] 邓娟,白洁.TRPC1和Ca~(2+)在帕金森病内质网应激中的作用[J].生命科学,2012,24(10):1169-1173. [43] MOCHIZUKI H, GOTO K, MORI H, et al. Histochemical detection of apoptosis in Parkinson’s disease. J Neurol Sci. 1996;137(2):120-123. [44] 钟河江,杨天德.内质网应激与免疫炎症反应的研究进展[J].重庆医学, 2012,41(2):201-203+206. [45] TAKAYANAGI S, FUKUDA R, TAKEUCHI Y,et al.Gene regulatory network of unfolded protein response genes in endoplasmic reticulum stress.Cell Stress Chaperones. 2013;18(1):11-23. [46] 陈欢.氯胺酮诱导PC12细胞凋亡的内质网应激机制研究[D].石家庄:河北医科大学,2014. [47] MCCULLOUGH K, MARTINDALE J, KLOTZ L,et al. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state.Mol Cell Biol. 2001;21(4):1249-1259. [48] 马骏,王中明,王述菊,等.电针对帕金森病模型大鼠内质网应激IRE1α-ASK1-JNK通路的影响[J].中国康复医学杂志,2018,33(6):658-662+680. [49] CRUZ J, TSAI L. Cdk5 deregulation in the pathogenesis of Alzheimer’s disease. Trends Mol Med. 2004;10(9):452-458. [50] MORSELLI E, MARIÑO G, BENNETZEN M, et al.Spermidine and resveratrol induce autophagy by distinct pathways converging on the acetylproteome. J Cell Biol. 2011;192(4):615-629. [51] 刘康永,石际俊,倪夏珺,等.α-核突触蛋白和自噬在帕金森病中的作用[J].中国临床神经科学,2010,18(1):103-107. [52] MASSEY AC, KAUSHIK S, SOVAK G,et al.Consequences of the selective blockage of chaperone-mediated autophagy. Proc Natl Acad Sci U S A. 2006;103(15):5805-5810. [53] LI D, SHI JJ, MAO CJ, et al.Alteration of dynein function affects α-synuclein degradation via the autophagosome-lysosome pathway. Int J Mol Sci. 2013; 14(12):24242-24254. [54] SHEN YF, TANG Y, ZHANG XJ,et al.Adaptive changes in autophagy after UPS impairment in Parkinson’s disease. Acta Pharmacol Sin. 2013;34(5):667-673. [55] Whitton P. Inflammation as a causative factor in the aetiology of Parkinson’s disease. Br J Pharmacol. 2007;150(8):963-976. [56] MORGANTI-KOSSMANN M, SATGUNASEELAN L, BYE N, et al: Modulation of immune response by head injury. Injury. 2007;38(12):1392-1400. [57] 吕风月,赵咏梅.星形胶质细胞在帕金森病发病机制中的作用[J].基础医学与临床,2008,28(5):516-518. [58] 庄文欣,付文玉,吕娥,等.帕金森病大鼠黑质小胶质细胞及星形胶质细胞的变化[J].解剖学报,2010,41(3):344-348. [59] 马骏,马彪,王述菊,等.电针对帕金森病大鼠纹状体缝隙连接蛋白43的表达及谷氨酸含量的影响[J].针刺研究,2015,40(5):364-367+372. [60] Visanji NP, Brooks PL, Hazrati LN,et al.The prion hypothesis in Parkinson’s disease: Braak to the future. Acta Neuropathol Commun. 2013;1:2. [61] Sampson TR, Debelius JW, Thron T,et al.Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease.Cell. 2016;167(6):1469-1480.e12. [62] Kim S, Kwon SH, Kam TI, et al.Transneuronal Propagation of Pathologic α-Synuclein from the Gut to the Brain Models Parkinson’s Disease. Neuron. 2019;103(4):627-641.e7. [63] 杨东明,杨利峰,赵德明,等.帕金森病动物模型的研究进展[J].中国实验动物学报,2020,28(3):397-404. [64] 陈蕾蕾,宋宁,谢俊霞.黑质铁沉积致帕金森病的机制研究进展[J].青岛大学学报(医学版),2020, 56(2):127-132. [65] 赵喆,鲍秀琦,张丹.铁死亡调控机制及其在帕金森病中的研究进展[J].药学学报,2019,54(3):399-406. |
| [1] | Wang Jianping, Zhang Xiaohui, Yu Jinwei, Wei Shaoliang, Zhang Xinmin, Xu Xingxin, Qu Haijun. Application of knee joint motion analysis in machanism based on three-dimensional image registration and coordinate transformation [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(在线): 1-5. |
| [2] | Tan Xinfang, Guo Yanxing, Qin Xiaofei, Zhang Binqing, Zhao Dongliang, Pan Kunkun, Li Yuzhuo, Chen Haoyu. Effect of uniaxial fatigue exercise on patellofemoral cartilage injury in a rabbit [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(在线): 1-6. |
| [3] | LIU Danni, SUN Guanghua, ZHOU Guijuan, LIU Hongya, ZHOU Jun, TAN Jinqu, HUANG Xiarong, PENG Ting, FENG Wei-bin, LUO Fu. Effect of electroacupuncture on apoptosis of neurons in cerebral cortex of rats with cerebral ischemia-reperfusion injury at "Shuigou" and "Baihui" points [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(在线): 1-6. |
| [4] | Bao Xianguo, Gao Zengxin, Wu Zhanpo, Chen Youmin, Cheng Qinghua, Lu Haitao, Guo Changzheng, Xu Shuai. Correlation between lumbar posterior muscle and local kyphosis in patients with degenerative thoracolumbar kyphosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1418-1423. |
| [5] | Zhu Chan, Han Xuke, Yao Chengjiao, Zhou Qian, Zhang Qiang, Chen Qiu. Human salivary components and osteoporosis/osteopenia [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1439-1444. |
| [6] | Zhang Haobo, Zhao Yunan, Yang Xuejun. Role and therapeutic implications of pyroptosis in intervertebral disc degeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1445-1451. |
| [7] | Jin Tao, Liu Lin, Zhu Xiaoyan, Shi Yucong, Niu Jianxiong, Zhang Tongtong, Wu Shujin, Yang Qingshan. Osteoarthritis and mitochondrial abnormalities [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1452-1458. |
| [8] | Zhang Lichuang, Xu Hao, Ma Yinghui, Xiong Mengting, Han Haihui, Bao Jiamin, Zhai Weitao, Liang Qianqian. Mechanism and prospects of regulating lymphatic reflux function in the treatment of rheumatoid arthritis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1459-1466. |
| [9] | Jing Jinpeng, Zhang Yue, Liu Xiaomin, Liu Yi. Traditional Chinese medicine injection for promoting blood circulation in prevention of deep vein thrombosis after orthopedic surgery: network meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1467-1476. |
| [10] | Li Huo, Wang Peng, Gao Jianming, Jiang Haoran, Lu Xiaobo, Peng Jiang. Relationship between revascularization and internal microstructure changes in osteonecrosis of the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1323-1328. |
| [11] | Gu Zhengqiu, Xu Fei, Wei Jia, Zou Yongdi, Wang Xiaolu, Li Yongming. Exploratory study on talk test as a measure of intensity in blood flow restriction training [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1154-1159. |
| [12] | Kong Yamin, Yan Juntao, Ma Bingxiang, Li Huawei. Massage vibration intervenes with MyoD expression and proliferation and differentiation of muscle satellite cells in rats with sciatic nerve injury [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1160-1166. |
| [13] | Wu Cong, Jia Quanzhong, Liu Lun. Relationship between transforming growth factor beta1 expression and chondrocyte migration in adult articular cartilage after fragmentation [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1167-1172. |
| [14] | Wang Baojuan, Zheng Shuguang, Zhang Qi, Li Tianyang. Miao medicine fumigation can delay extracellular matrix destruction in a rabbit model of knee osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1180-1186. |
| [15] | Lü Yiyan, Li Hanbing, Ma Xiaoqing, Zhang Han, Zhang Yuhang, Li Genlin. Establishment and characteristic analysis of interior heat and diabetes mouse model using compound factors [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1187-1193. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||