Chinese Journal of Tissue Engineering Research ›› 2022, Vol. 26 ›› Issue (25): 3944-3950.doi: 10.12307/2022.396
Previous Articles Next Articles
Tail vein injection of bone marrow mesenchymal stem cells for repair of skull injury in aging mice
Zhang Jingying1, Li Ziyi1, Liu Xiaochuan1, Li Dan1, Wang Yang2, Wu zhuguo1
- 1The First Dongguan Affiliated Hospital of Guangdong Medical University, Dongguan 523808, Guangdong Province, China; 2Department of Endocrinology, Huludao Central Hospital, Huludao 125000, Liaoning Province, China
-
Received:2021-02-27Accepted:2021-04-15Online:2022-09-08Published:2022-01-25 -
Contact:Zhang Jingying, PhD, Associate professor, The First Dongguan Affiliated Hospital of Guangdong Medical University, Dongguan 523808, Guangdong Province, China -
About author:Zhang Jingying, PhD, Associate professor, The First Dongguan Affiliated Hospital of Guangdong Medical University, Dongguan 523808, Guangdong Province, China -
Supported by:the Basic and Applied Basic Research of Guangdong Province, No. 2020B1515120001 (to ZJY); Key Fields Foundation of Colleges and Universities in Guangdong Province, No. 2020ZDZX2013 (to ZJY); Discipline Construction Project of Guangdong Medical University, No. 4SG21019G (to ZJY), No. 4SG21015G (to WZG)
CLC Number:
Cite this article
Zhang Jingying, Li Ziyi, Liu Xiaochuan, Li Dan, Wang Yang, Wu zhuguo. Tail vein injection of bone marrow mesenchymal stem cells for repair of skull injury in aging mice[J]. Chinese Journal of Tissue Engineering Research, 2022, 26(25): 3944-3950.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks

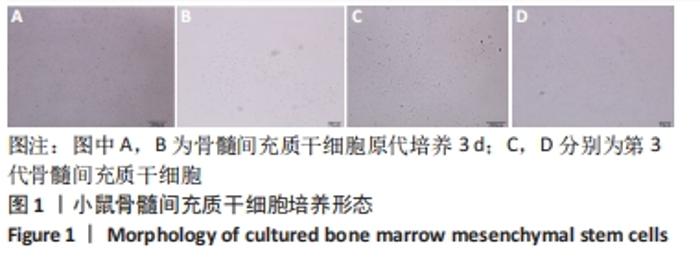
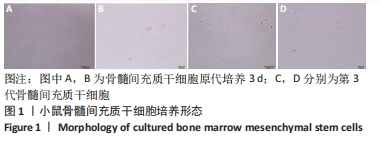
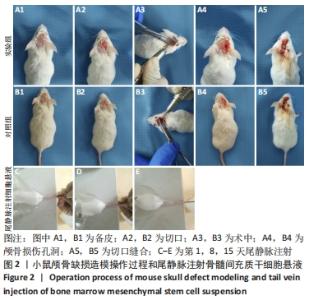
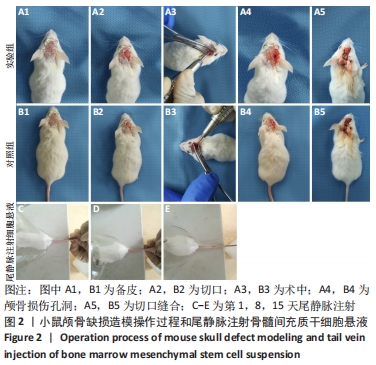
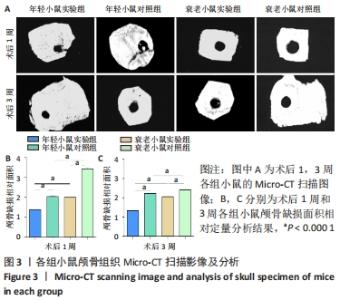
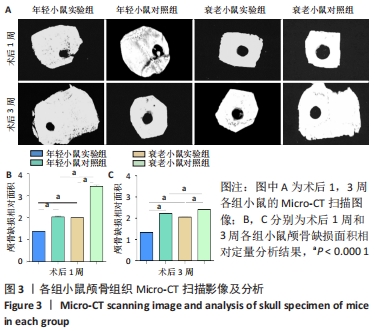
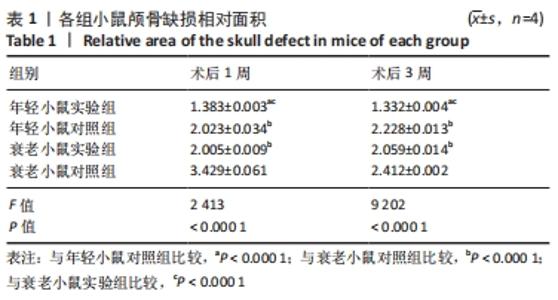
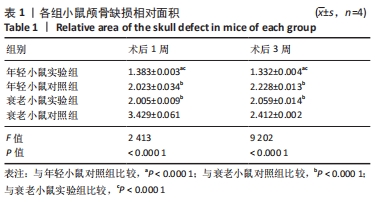
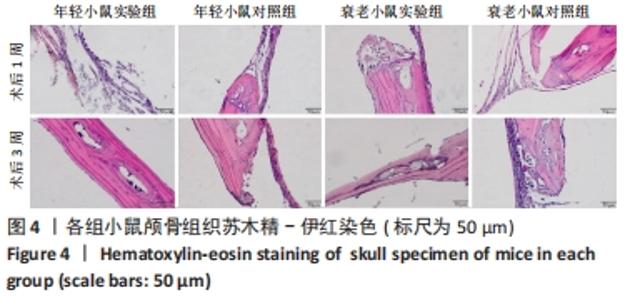
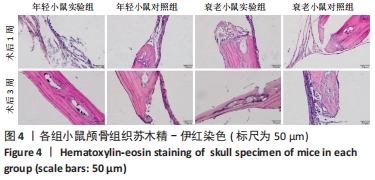
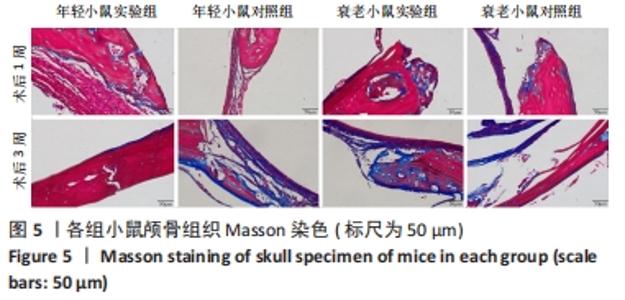
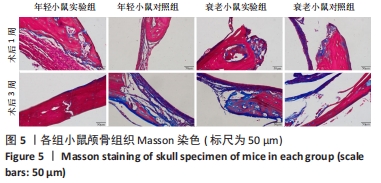
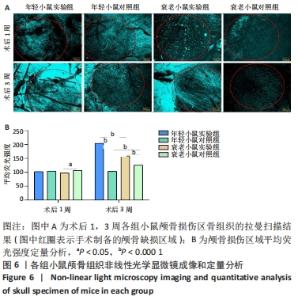
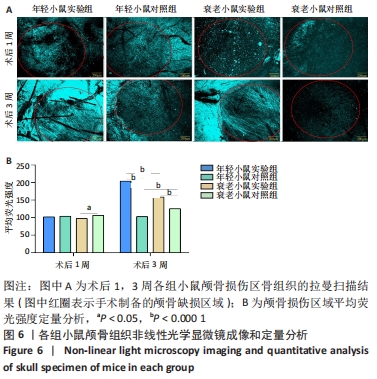
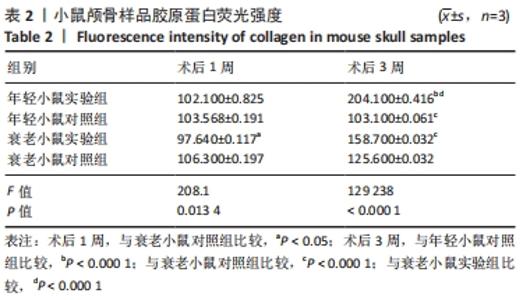
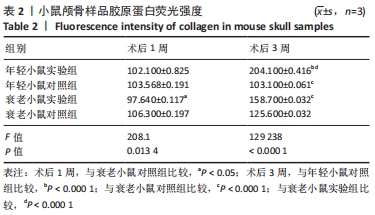
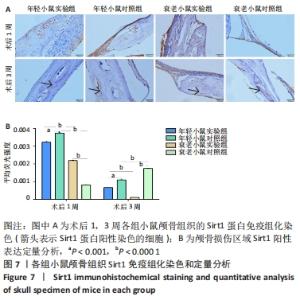
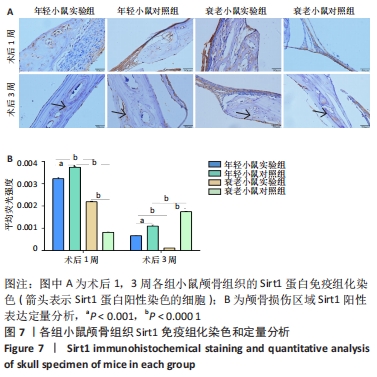
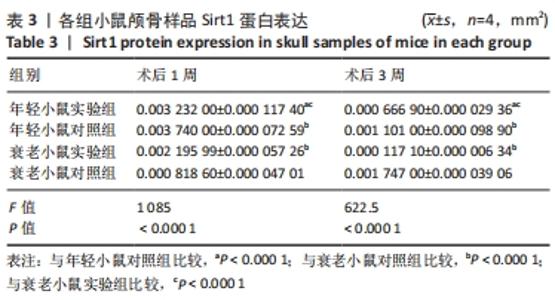
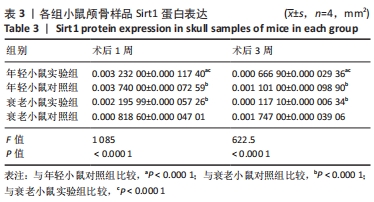
术后1周,各组骨缺损处均出现了棕黄色Sirt1蛋白阳性表达(图7红色箭头所指棕黄色区域),通过ImageJ软件处理图像可得出阳性区域面积,术后1周,相同年龄段小鼠对比,衰老小鼠实验组Sirt1蛋白表达高于衰老小鼠对照组(P < 0.000 1);而年轻小鼠实验组的Sirt1蛋白表达低于年轻小鼠对照组(P < 0.001);不同年龄段小鼠对比,年轻小鼠的Sirt1蛋白表达高于衰老小鼠(P < 0.000 1);术后3周,各组Sirt1蛋白阳性表达降低,相同年龄段(1月龄或12月龄)小鼠实验组Sirt1蛋白表达低于对照组(P < 0.001-0.000 1);对于不同年龄段而言,年轻小鼠实验组Sirt1蛋白表达高于衰老小鼠实验组(P < 0.000 1),而年轻小鼠对照组Sirt1蛋白含量低于衰老小鼠对照组(P < 0.001-0.000 1)。 2.8 生物相容性 间充质干细胞因具有多向分化潜能、免疫原性低等优势,成为细胞治疗和组织工程的研究热点。研究证实,将异体骨髓间充质干细胞通过肌肉和尾静脉途径注射到大鼠体内,大鼠未出现明显的炎症及免疫反应,表现出较好的组织相容性[11-13]。"

| [1] APOSTU D, LUCACIU O, MESTER A, et al. Cannabinoids and bone regeneration. Drug Metab Rev. 2019;51(1):65-75. [2] PERIĆ KAČAREVIĆ Ž, RIDER P, ALKILDANI S, et al. An introduction to bone tissue engineering. Int J Artif Organs. 2020;43(2):69-86. [3] LEE J, BYUN H, MADHURAKKAT PERIKAMANA SK, et al. Current Advances in Immunomodulatory Biomaterials for Bone Regeneration. Adv Healthc Mater. 2019;8(4):e1801106. [4] LIN H, SOHN J, SHEN H, et al. Bone marrow mesenchymal stem cells: Aging and tissue engineering applications to enhance bone healing. Biomaterials. 2019;203:96-110. [5] GIBON E, LU L, GOODMAN SB. Aging, inflammation, stem cells, and bone healing. Stem Cell Res Ther. 2016;7:44. [6] RIZZOLI R, REGINSTER JY, BOONEN S, et al. Adverse reactions and drug-drug interactions in the management of women with postmenopausal osteoporosis. Calcif Tissue Int. 2011;89(2):91-104. [7] ROZMAN C, FELIU E, BERGA L, et al. Age-related variations of fat tissue fraction in normal human bone marrow depend both on size and number of adipocytes: a stereological study. Exp Hematol. 1989;17(1):34-37. [8] BUNPETCH V, ZHANG ZY, ZHANG X, et al. Strategies for MSC expansion and MSC-based microtissue for bone regeneration. Biomaterials. 2019;196:67-79. [9] PAJARINEN J, LIN T, GIBON E, et al. Mesenchymal stem cell-macrophage crosstalk and bone healing. Biomaterials. 2019;196:80-89. [10] HORWITZ EM, PROCKOP DJ, GORDON PL, et al. Clinical responses to bone marrow transplantation in children with severe osteogenesis imperfecta. Blood. 2001;97(5):1227-1231. [11] LI P, MA X, JIN W, et al. Effects of local injection and intravenous injection of allogeneic bone marrow mesenchymal stem cells on the structure and function of damaged anal sphincter in rats. J Tissue Eng Regen Med. 2020;14(7):989-1000. [12] HAO C, WANG Y, SHAO L, et al. Local Injection of Bone Mesenchymal Stem Cells and Fibrin Glue Promotes the Repair of Bone Atrophic Nonunion In Vivo. Adv Ther. 2016;33(5):824-833. [13] ZHANG R, MA J, HAN J, et al. Mesenchymal stem cell related therapies for cartilage lesions and osteoarthritis. Am J Transl Res. 2019;11(10):6275-6289. [14] MAJIDINIA M, SADEGHPOUR A, YOUSEFI B. The roles of signaling pathways in bone repair and regeneration. J Cell Physiol. 2018;233(4):2937-2948. [15] O’BRIEN FJ. Biomaterials & scaffolds for tissue engineering. Materials Today. 2011;14(3):88-95. [16] WANG MO, VORWALD CE, DREHER ML, et al. Evaluating 3D-printed biomaterials as scaffolds for vascularized bone tissue engineering. Adv Mater. 2015;27(1):138-144. [17] SHICK TM, KADIR AZA,NGADIMAN NHA,et al.A review of biomaterials scaffold fabrication in additive manufacturing for tissue engineering. J Bioact Compat Polym. 2019;34(6):415-435. [18] REN X, FENG Y, GUO J, et al. Surface modification and endothelialization of biomaterials as potential scaffolds for vascular tissue engineering applications. Chem Soc Rev. 2015;44(15):5680-5742. [19] BARBA A, MAAZOUZ Y, DIEZ-ESCUDERO A, et al. Osteogenesis by foamed and 3D-printed nanostructured calcium phosphate scaffolds: Effect of pore architecture. Acta Biomater. 2018;79:135-147. [20] BOEHM AV, MEININGER S, TESCH A, et al. The Mechanical Properties of Biocompatible Apatite Bone Cement Reinforced with Chemically Activated Carbon Fibers. Materials (Basel). 2018;11(2):192. [21] CHEN Y, LIU X, LIU R, et al. Zero-order controlled release of BMP2-derived peptide P24 from the chitosan scaffold by chemical grafting modification technique for promotion of osteogenesis in vitro and enhancement of bone repair in vivo. Theranostics. 2017;7(5):1072-1087. [22] 庄传记,陈文昭,江新民.骨形态发生蛋白9复合胶原基骨修复材料体外成骨性能与体内修复骨缺损[J].中国组织工程研究,2021,25(10): 1489-1494. [23] DA COSTA JP, VITORINO R, SILVA GM, et al. A synopsis on aging-Theories, mechanisms and future prospects. Ageing Res Rev. 2016;29:90-112. [24] OTSUKA K, MURAKAMI S, KUBO Y, et al. Chronomics for chronoastrobiology with immediate spin-offs for life quality and longevity. Biomed Pharmacother. 2003;57 Suppl 1:1s-18s. [25] WANG Z, SHI Y, CHEN W, et al. Mesenchymal stem cells repair bone marrow damage of aging rats and regulate autophagy and aging genes. Cell Biochem Funct. 2020;38(6):792-800. [26] GOODELL MA, RANDO TA. Stem cells and healthy aging. Science. 2015; 350(6265):1199-1204. [27] WANG MJ, CHEN J, CHEN F, et al. Rejuvenating Strategies of Tissue-specific Stem Cells for Healthy Aging. Aging Dis. 2019;10(4):871-882. [28] OSUGI M, KATAGIRI W, YOSHIMI R, et al. Conditioned media from mesenchymal stem cells enhanced bone regeneration in rat calvarial bone defects. Tissue Eng Part A. 2012;18(13-14):1479-1489. [29] 袁宇,徐林.骨髓间充质干细胞联合3D生物打印技术治疗骨缺损的研究进展[J].中国医学物理学杂志,2021,38(1):110-126. [30] 刘相杰,宋科官.生物支架材料及间充质干细胞在骨组织工程中的研究与应用[J].中国组织工程研究,2018,22(10):1618-1624. [31] DHIVYA S, KESHAV NARAYAN A, Logith Kumar R, et al. Proliferation and differentiation of mesenchymal stem cells on scaffolds containing chitosan, calcium polyphosphate and pigeonite for bone tissue engineering. Cell Prolif. 2018;51(1):e12408. [32] CHEN W, LIU J, MANUCHEHRABADI N, et al. Umbilical cord and bone marrow mesenchymal stem cell seeding on macroporous calcium phosphate for bone regeneration in rat cranial defects. Biomaterials. 2013;34(38):9917-9925. [33] HANG HL, XIA Q. Role of BMSCs in liver regeneration and metastasis after hepatectomy. World J Gastroenterol. 2014;20(1):126-132. [34] 魏琴,张雪,马磊,等.血小板衍生生长因子BB诱导大鼠骨髓间充质干细胞向成骨细胞分化[J].中国组织工程研究,2021,25(19):2953-2957. [35] 姜涛,吴硕,李志强,等.血小板衍生生长因子BB促进SD大鼠骨髓间充质干细胞的增殖[J].中国组织工程研究,2021,25(13):1976-1981. [36] LI X, WANG Y, AN G, et al. Bone marrow mesenchymal stem cells attenuate silica-induced pulmonary fibrosis via paracrine mechanisms. Toxicol Lett. 2017;270:96-107. [37] TSAI MJ, TSAI SK, HU BR, et al. Recovery of neurological function of ischemic stroke by application of conditioned medium of bone marrow mesenchymal stem cells derived from normal and cerebral ischemia rats. J Biomed Sci. 2014;21(1):5. [38] Sun W, Qiao W, Zhou B, et al. Overexpression of Sirt1 in mesenchymal stem cells protects against bone loss in mice by FOXO3a deacetylation and oxidative stress inhibition. Metabolism. 2018;88:61-71. [39] LIU J, WANG H, REN W, et al. β-mercaptoethanol promotes osteogenesis of human mesenchymal stem cells via sirt1-ERK pathway. Cytotechnology. 2020;72(5):695-706. [40] YANG X, JIANG T, WANG Y, et al. The Role and Mechanism of SIRT1 in Resveratrol-regulated Osteoblast Autophagy in Osteoporosis Rats. Sci Rep. 2019;9(1):18424. [41] JIANG Y, LUO W, WANG B, et al. Resveratrol promotes osteogenesis via activating SIRT1/FoxO1 pathway in osteoporosis mice. Life Sci. 2020;246: 117422. [42] 王娜,陈乃耀. SIRT1影响间充质干细胞骨修复的研究进展[J].基础医学与临床,2014,34(12):1714-1717. |
| [1] | Pan Baoshun, Fang Zhen, Gao Mingjie, Fang Guiming, Chen Jinshui. Design for posterior atlantoaxial internal fixation system with fusion cage based on imaging data [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1372-1376. |
| [2] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [3] | Wang Jing, Xiong Shan, Cao Jin, Feng Linwei, Wang Xin. Role and mechanism of interleukin-3 in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1260-1265. |
| [4] | Xiao Hao, Liu Jing, Zhou Jun. Research progress of pulsed electromagnetic field in the treatment of postmenopausal osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1266-1271. |
| [5] | Huang Chenwei, Fei Yankang, Zhu Mengmei, Li Penghao, Yu Bing. Important role of glutathione in stemness and regulation of stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1119-1124. |
| [6] | Hui Xiaoshan, Bai Jing, Zhou Siyuan, Wang Jie, Zhang Jinsheng, He Qingyong, Meng Peipei. Theoretical mechanism of traditional Chinese medicine theory on stem cell induced differentiation [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1125-1129. |
| [7] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [8] | Fan Yiming, Liu Fangyu, Zhang Hongyu, Li Shuai, Wang Yansong. Serial questions about endogenous neural stem cell response in the ependymal zone after spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1137-1142. |
| [9] | Wen Dandan, Li Qiang, Shen Caiqi, Ji Zhe, Jin Peisheng. Nocardia rubra cell wall skeleton for extemal use improves the viability of adipogenic mesenchymal stem cells and promotes diabetes wound repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1038-1044. |
| [10] | Zhu Bingbing, Deng Jianghua, Chen Jingjing, Mu Xiaoling. Interleukin-8 receptor enhances the migration and adhesion of umbilical cord mesenchymal stem cells to injured endothelium [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1045-1050. |
| [11] | Luo Xiaoling, Zhang Li, Yang Maohua, Xu Jie, Xu Xiaomei. Effect of naringenin on osteogenic differentiation of human periodontal ligament stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1051-1056. |
| [12] | Wang Xinmin, Liu Fei, Xu Jie, Bai Yuxi, Lü Jian. Core decompression combined with dental pulp stem cells in the treatment of steroid-associated femoral head necrosis in rabbits [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1074-1079. |
| [13] | Fang Xiaolei, Leng Jun, Zhang Chen, Liu Huimin, Guo Wen. Systematic evaluation of different therapeutic effects of mesenchymal stem cell transplantation in the treatment of ischemic stroke [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1085-1092. |
| [14] | Guo Jia, Ding Qionghua, Liu Ze, Lü Siyi, Zhou Quancheng, Gao Yuhua, Bai Chunyu. Biological characteristics and immunoregulation of exosomes derived from mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1093-1101. |
| [15] | Tian Chuan, Zhu Xiangqing, Yang Zailing, Yan Donghai, Li Ye, Wang Yanying, Yang Yukun, He Jie, Lü Guanke, Cai Xuemin, Shu Liping, He Zhixu, Pan Xinghua. Bone marrow mesenchymal stem cells regulate ovarian aging in macaques [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 985-991. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||