Chinese Journal of Tissue Engineering Research ›› 2022, Vol. 26 ›› Issue (24): 3846-3851.doi: 10.12307/2022.564
Previous Articles Next Articles
Osteogenic differentiation of bone marrow mesenchymal stem cells in obese mice
Chen Chichi1, 2, Zhang Yu1, 2, He Jiachen1, 2, Shi Qin1, 2
- 1Medical College of Soochow University, Department of Orthopedics, First Affiliated Hospital of Soochow University, Suzhou 215123, Jiangsu Province, China; 2Institute of Orthopedics, Soochow University, Suzhou 215006, Jiangsu Province, China
-
Received:2021-03-13Accepted:2021-04-10Online:2022-08-28Published:2022-01-22 -
Contact:Shi Qin, PhD, Researcher, Medical College of Soochow University, Department of Orthopedics, First Affiliated Hospital of Soochow University, Suzhou 215123, Jiangsu Province, China; Institute of Orthopedics, Soochow University, Suzhou 215006, Jiangsu Province, China -
About author:Chen Chichi, Medical College of Soochow University, Department of Orthopedics, First Affiliated Hospital of Soochow University, Suzhou 215123, Jiangsu Province, China; Institute of Orthopedics, Soochow University, Suzhou 215006, Jiangsu Province, China -
Supported by:the National Natural Science Foundation of China, No. 81972059 (to SQ)
CLC Number:
Cite this article
Chen Chichi, Zhang Yu, He Jiachen, Shi Qin. Osteogenic differentiation of bone marrow mesenchymal stem cells in obese mice[J]. Chinese Journal of Tissue Engineering Research, 2022, 26(24): 3846-3851.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
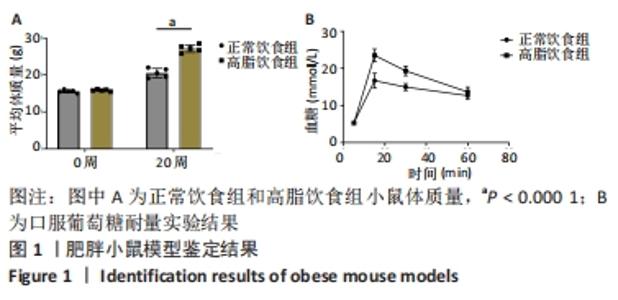
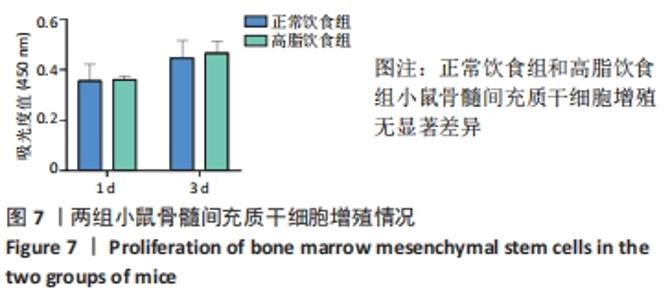
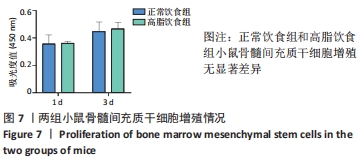
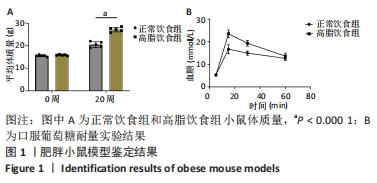
2.1 实验动物数量分析 4-6周龄雌性Balb/c小鼠16只,适应饲养1周后,随机均分为2组:正常饮食组和高脂饮食组,每组8只,其中3只用来提取骨髓间充质干细胞进行细胞实验,剩余5只小鼠先进行Micro-CT分析然后进行组织形态学染色,中途无脱落。 2.2 肥胖小鼠模型的鉴定结果 小鼠饲喂高脂饮食饲料之前,正常饮食组和高脂饮食组小鼠体质量差异无显著性意义,见图1A;高脂饲料饲养20周之后,与正常饮食组相比,高脂饮食组小鼠体质量均显著增加,差异有显著性意义。在糖代谢方面,葡萄糖耐量实验结果显示高脂饮食组小鼠的血糖水平在口服葡萄糖15 min时显著高于正常饮食组,见图1B,说明高脂饮食导致的小鼠肥胖模型构建成功。"
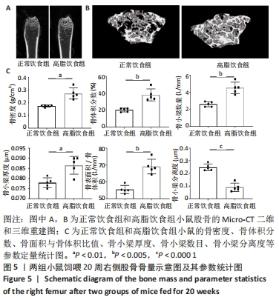
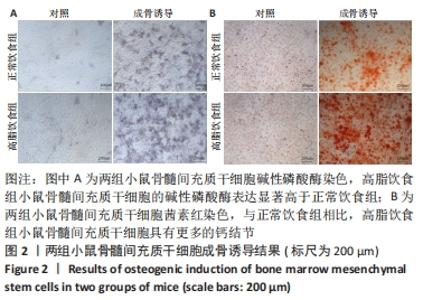
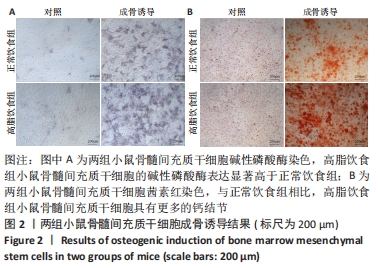
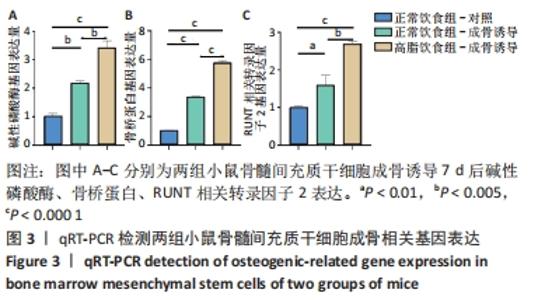
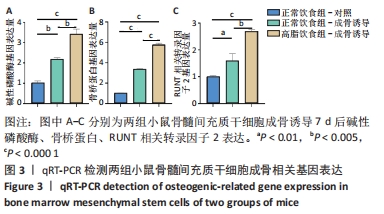
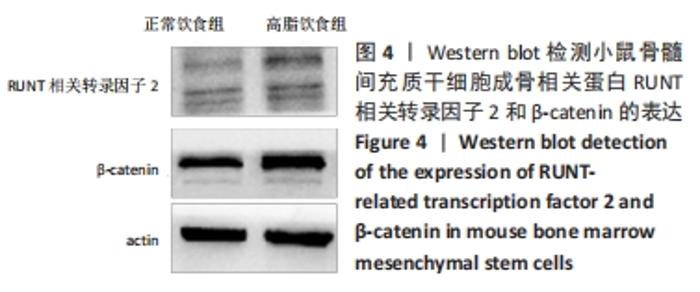
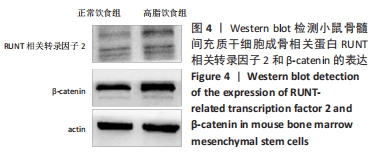
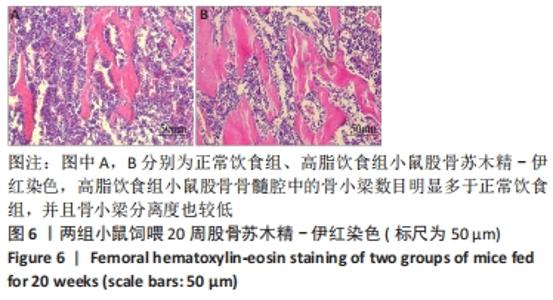
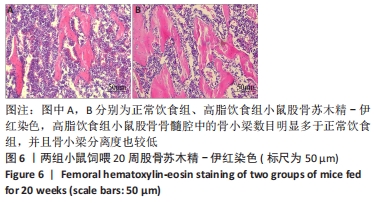
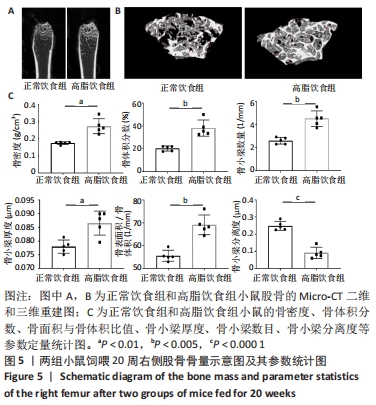
2.6 两组小鼠股骨Micro-CT分析结果 两组小鼠股骨的二维和三维重建图像清晰地显示:与正常饮食组小鼠相比,高脂饮食组小鼠股骨中骨小梁比较密集,骨小梁间隙也明显减小,说明高脂饮食组小鼠骨量较多,见图5A,B。通过分析小鼠股骨相关参数发现:与正常饮食组小鼠相比,高脂饮食组小鼠骨密度显著增加[正常饮食组:(0.174±0.008) g/cm3,高脂饮食组:(0.273±0.044) g/cm3],差异有显著性意义;正常饮食组小鼠的骨体积分数为(20.305±2.212)%,高脂饮食组为(38.125±7.068)%,比正常饮食组增加了87.76%,差异有显著性意义;正常饮食组小鼠骨面积与骨体积比值为(55.572±2.409) 1/mm,高脂饮食组为(69.321±4.961) 1/mm,比正常饮食组增加了24.74%,差异有显著性意义;正常饮食组的骨小梁厚度为(0.078±0.002) μm,高脂饮食组为(0.087±0.004) μm,比正常饮食组增加了11.54%,差异有显著性意义;正常饮食组和高脂饮食组的骨小梁数目分别为(2.606±0.292)1/mm和(4.51±0.685) 1/mm,高脂饮食组增加了73.06%,差异有显著性意义;正常饮食组和高脂饮食组的骨小梁分离度分别为(0.249±0.026) μm和(0.091±0.033) μm,高脂饮食组减少了63.45%,差异有显著性意义。这些效应表明高脂饮食组小鼠的骨密度、骨小梁数目和骨小梁厚度均增加,骨小梁分离度减少,说明高脂饮食组小鼠骨形成增强,见图5C。"
| [1] GBD 2015 OBESITY COLLABORATORS, AFSHIN A, FOROUZANFAR MH, et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years.N Engl J Med. 2017;377(1):13-27. [2] GREGG EW, SHAW JE. Global Health Effects of Overweight and Obesity. N Engl J Med. 2017;377(1):80-81. [3] 董虹孛,闫银坤,米杰.儿童肥胖与骨量的双重性关系[J].中华骨质疏松症和骨矿盐疾病杂志,2019,12(4):406-412. [4] 王烁,董彦会,王政和,等. 1985—2014 年中国7~18 岁学生超重与肥胖流行趋势[J].中华预防医学杂志,2017,51(4):300-305. [5] EVANS AL, PAGGIOSI MA, EASTELL R, et al. Bone density, microstructure and strength in obese and normal weight men and women in younger and older adulthood. J Bone Miner Res. 2015;30(5):920-928. [6] ARMSTRONG ME, SPENCER EA, CAIRNS BJ, et al. Body mass index and physical activity in relation to the incidence of hip fracture in postmenopausal women. J Bone Miner Res. 2011;26(6):1330-1338. [7] ARMSTRONG ME, CAIRNS BJ, BANKS E, et al. Different effects of age, adiposity and physical activity on the risk of ankle, wrist and hip fractures in postmenopausal women. Bone. 2012;50(6):1394-1400. [8] PRIETO-ALHAMBRA D, PREMAOR MO, FINA AVILÉS F, et al. The association between fracture and obesity is site-dependent: a population-based study in postmenopausal women. J Bone Miner Res. 2012;27(2):294-300. [9] PREMAOR MO, COMPSTON JE, FINA AVILÉS F, et al. The association between fracture site and obesity in men: a population-based cohort study. J Bone Miner Res. 2013;28(8):1771-1777. [10] DE LAET C, KANIS JA, ODÉN A, et al. Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporos Int. 2005;16(11):1330-1338. [11] JOHANSSON H, KANIS JA, ODÉN A, et al. A meta-analysis of the association of fracture risk and body mass index in women. J Bone Miner Res. 2014;29(1):223-233. [12] GNUDI S, SITTA E, LISI L. Relationship of body mass index with main limb fragility fractures in postmenopausal women. J Bone Miner Metab. 2009;27(4):479-484. [13] BECK TJ, PETIT MA, WU G, et al. Does obesity really make the femur stronger? BMD, geometry, and fracture incidence in the women’s health initiative-observational study. J Bone Miner Res. 2009;24(8): 1369-1379. [14] COMPSTON JE, WATTS NB, CHAPURLAT R, et al. Obesity is not protective against fracture in postmenopausal women: GLOW. Am J Med. 2011;124(11):1043-1050. [15] ESTRADA A, RAMNITZ MS, GAFNI RI. Bone densitometry in children and adolescents. Curr Opin Obstet Gynecol. 2014;26(5):339-346. [16] BRONCKERS AL, SASAGURI K, ENGELSE MA. Transcription and immunolocalization of Runx2/Cbfa1/Pebp2alphaA in developing rodent and human craniofacial tissues: further evidence suggesting osteoclasts phagocytose osteocytes. Microsc Res Tech. 2003;61(6):540-548. [17] HORWITZ EM, LE BLANC K, DOMINICI M, et al. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy. 2005;7(5):393-395. [18] QADIR A, LIANG S, WU Z, et al. Senile Osteoporosis: The Involvement of Differentiation and Senescence of Bone Marrow Stromal Cells. Int J Mol Sci. 2020;21(1):349. [19] GAO J, XIANG S, WEI X, et al. Icariin Promotes the Osteogenesis of Bone Marrow Mesenchymal Stem Cells through Regulating Sclerostin and Activating the Wnt/β-Catenin Signaling Pathway. Biomed Res Int. 2021;2021:6666836. [20] SI L, WINZENBERG TM, JIANG Q, et al. Projection of osteoporosis-related fractures and costs in China: 2010-2050. Osteoporos Int. 2015; 26(7):1929-1937. [21] SI L, WINZENBERG TM, DE GRAAFF B, et al. A systematic review and meta-analysis of utility-based quality of life for osteoporosis-related conditions. Osteoporos Int. 2014;25(8):1987-1997. [22] ZHOU W, LIN J, ZHAO K, et al. Single-Cell Profiles and Clinically Useful Properties of Human Mesenchymal Stem Cells of Adipose and Bone Marrow Origin. Am J Sports Med. 2019;47(7):1722-1733. [23] DELANOIS RE, ETCHESON JI, SODHI N, et al. Biologic Therapies for the Treatment of Knee Osteoarthritis. J Arthroplasty. 2019;34(4):801-813. [24] FROST HM. The mechanostat: a proposed pathogenic mechanism of osteoporoses and the bone mass effects of mechanical and nonmechanical agents. Bone Miner. 1987;2(2):73-85. [25] FROST HM. Bone’s mechanostat: a 2003 update. Anat Rec A Discov Mol Cell Evol Biol. 2003;275(2):1081-1101. [26] KINDLER JM, LEWIS RD, HAMRICK MW. Skeletal muscle and pediatric bone development. Curr Opin Endocrinol Diabetes Obes. 2015;22(6): 467-474. [27] VAN LEEUWEN J, KOES BW, PAULIS WD, et al. Differences in bone mineral density between normal-weight children and children with overweight and obesity: a systematic review and meta-analysis. Obes Rev. 2017;18(5):526-546. [28] CLARK EM, NESS AR, TOBIAS JH. Adipose tissue stimulates bone growth in prepubertal children. J Clin Endocrinol Metab. 2006;91(7):2534-2541. [29] UUSI-RASI K, LAAKSONEN M, MIKKILÄ V, et al. Overweight in childhood and bone density and size in adulthood. Osteoporos Int. 2012;23(4): 1453-1461. [30] WHITING SJ, VATANPARAST H, BAXTER-JONES A, et al. Factors that affect bone mineral accrual in the adolescent growth spurt. J Nutr. 2004;134(3):696S-700S. [31] MAÏMOUN L, COSTE O, MURA T, et al. Specific bone mass acquisition in elite female athletes. J Clin Endocrinol Metab. 2013;98(7):2844-2853. [32] BAILEY DA, MCKAY HA, MIRWALD RL, et al. A six-year longitudinal study of the relationship of physical activity to bone mineral accrual in growing children: the university of Saskatchewan bone mineral accrual study. J Bone Miner Res. 1999;14(10):1672-1679. [33] BONJOUR JP, THEINTZ G, BUCHS B, et al. Critical years and stages of puberty for spinal and femoral bone mass accumulation during adolescence. J Clin Endocrinol Metab. 1991;73(3):555-563. [34] HENRY YM, FATAYERJI D, EASTELL R. Attainment of peak bone mass at the lumbar spine, femoral neck and radius in men and women: relative contributions of bone size and volumetric bone mineral density. Osteoporos Int. 2004;15(4):263-273. [35] VILJAKAINEN H, IVASKA KK, PALDÁNIUS P, et al. Suppressed bone turnover in obesity: a link to energy metabolism? A case-control study. J Clin Endocrinol Metab. 2014;99(6):2155-2163. [36] KANG C, LEROITH D, GALLAGHER EJ. Diabetes, Obesity, and Breast Cancer. Endocrinology. 2018;159(11):3801-3812. [37] PROTANI M, COORY M, MARTIN JH. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat. 2010;123(3):627-635. [38] IKEDA K, TAKESHITA S. The role of osteoclast differentiation and function in skeletal homeostasis. J Biochem. 2016;159(1):1-8. [39] PAJARINEN J, LIN T, GIBON E, et al. Mesenchymal stem cell-macrophage crosstalk and bone healing. Biomaterials. 2019;196:80-89. |
| [1] | Wang Jing, Xiong Shan, Cao Jin, Feng Linwei, Wang Xin. Role and mechanism of interleukin-3 in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1260-1265. |
| [2] | Xiao Hao, Liu Jing, Zhou Jun. Research progress of pulsed electromagnetic field in the treatment of postmenopausal osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1266-1271. |
| [3] | Wen Dandan, Li Qiang, Shen Caiqi, Ji Zhe, Jin Peisheng. Nocardia rubra cell wall skeleton for extemal use improves the viability of adipogenic mesenchymal stem cells and promotes diabetes wound repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1038-1044. |
| [4] | Zhu Bingbing, Deng Jianghua, Chen Jingjing, Mu Xiaoling. Interleukin-8 receptor enhances the migration and adhesion of umbilical cord mesenchymal stem cells to injured endothelium [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1045-1050. |
| [5] | Luo Xiaoling, Zhang Li, Yang Maohua, Xu Jie, Xu Xiaomei. Effect of naringenin on osteogenic differentiation of human periodontal ligament stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1051-1056. |
| [6] | Wang Xinmin, Liu Fei, Xu Jie, Bai Yuxi, Lü Jian. Core decompression combined with dental pulp stem cells in the treatment of steroid-associated femoral head necrosis in rabbits [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1074-1079. |
| [7] | Fang Xiaolei, Leng Jun, Zhang Chen, Liu Huimin, Guo Wen. Systematic evaluation of different therapeutic effects of mesenchymal stem cell transplantation in the treatment of ischemic stroke [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1085-1092. |
| [8] | Guo Jia, Ding Qionghua, Liu Ze, Lü Siyi, Zhou Quancheng, Gao Yuhua, Bai Chunyu. Biological characteristics and immunoregulation of exosomes derived from mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1093-1101. |
| [9] | Huang Chenwei, Fei Yankang, Zhu Mengmei, Li Penghao, Yu Bing. Important role of glutathione in stemness and regulation of stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1119-1124. |
| [10] | Hui Xiaoshan, Bai Jing, Zhou Siyuan, Wang Jie, Zhang Jinsheng, He Qingyong, Meng Peipei. Theoretical mechanism of traditional Chinese medicine theory on stem cell induced differentiation [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1125-1129. |
| [11] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [12] | Fan Yiming, Liu Fangyu, Zhang Hongyu, Li Shuai, Wang Yansong. Serial questions about endogenous neural stem cell response in the ependymal zone after spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1137-1142. |
| [13] | Tian Chuan, Zhu Xiangqing, Yang Zailing, Yan Donghai, Li Ye, Wang Yanying, Yang Yukun, He Jie, Lü Guanke, Cai Xuemin, Shu Liping, He Zhixu, Pan Xinghua. Bone marrow mesenchymal stem cells regulate ovarian aging in macaques [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 985-991. |
| [14] | Gao Yujin, Peng Shuanglin, Ma Zhichao, Lu Shi, Cao Huayue, Wang Lang, Xiao Jingang. Osteogenic ability of adipose stem cells in diabetic osteoporosis mice [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 999-1004. |
| [15] | Hou Jingying, Guo Tianzhu, Yu Menglei, Long Huibao, Wu Hao. Hypoxia preconditioning targets and downregulates miR-195 and promotes bone marrow mesenchymal stem cell survival and pro-angiogenic potential by activating MALAT1 [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1005-1011. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||