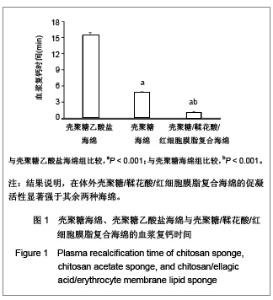
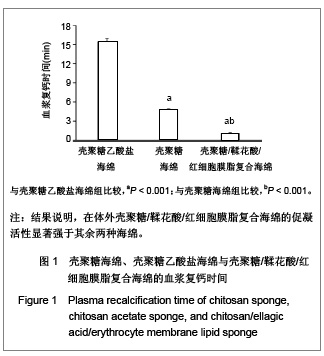
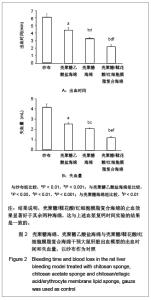
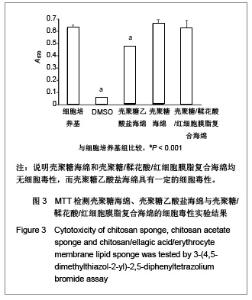
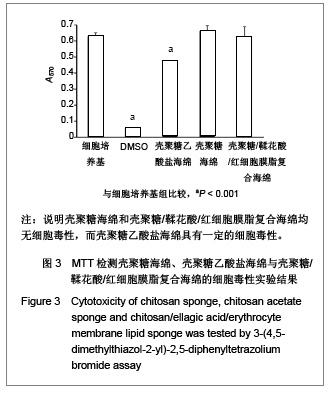
| [1] He Q,Ao Q,Gong K,et al.Preparation and characterization of chitosan-heparin composite matrices for blood contacting tissue engineering. Biomed Mater. 2010;5(5):055001.[2] Ao Q,Wang A,Cao W,et al. Manufacture of multimicrotubule chitosan nerve conduits with novel molds and characterization in vitro.J Biomed Mater Res A. 2006;77(1):11-18.[3] Malette WG,Quigley HJ,Gaines RD,et al. Chitosan: a new hemostatic. Ann Thorac Surg. 1983;36(1):55-58.[4] Yang J,Tian F,Wang Z,et al.Effect of chitosan molecular weight and deacetylation degree on hemostasis. J Biomed Mater Res B. 2008;84(1):131-137.[5] Peng T. Biomaterials for hemorrhage control. Trends Biomater Artif Organs. 2010;24(1):27-68.[6] Englehart MS,Cho SD,Tieu BH,et al.A novel highly porous silica and chitosan-based hemostatic dressing is superior to HemCon and gauze sponges. J Trauma. 2008;65(4):884-890.[7] Vekiari SA,Gordon MH,Garcia-Macias P,et al. Extraction and determination of ellagic acid content in chestnut bark and fruit.Food Chem. 2008;110(4):1007-1011.[8] Damas J,Remacle-Volon G,Adam A.Some cardiovascular and hematological changes induced in the rat by activation of Hageman factor with ellagic acid. Adv Exp Med Biol.1989; 247A:461-465.[9] Zhou J,Zheng Y,Shi J,et al. Daunorubicin induces procoagulant response through phosphatidylserine exposure in red blood cells.Thromb Res. 2010;125(2):178-183.[10] The Ministry of Science and Technology of the People’s Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30. 中华人民共和国科学技术部.关于善待实验动物的指导性意见. 2006-09-30.[11] Mawatari S,Yunoki K,Sugiyama M,et al. Simultaneous preparation of purified plasmalogens and sphingomyelin in human erythrocytes with phospholipase A1 from Aspergillus orizae. Biosci Biotechnol Biochem. 2009;73(12):2621-2625.[12] Liu C,Yang S,Liu W,et al.Preparation and characterization of medium-chain fatty acid liposomes by lyophilization. J Liposome Res. 2010;20(3):183-190.[13] Tuzlakoglu K,Alves CM,Mano JF,et al.Production and characterization of chitosan fibers and 3-D fiber mesh scaffolds for tissue engineering applications.Macromol Biosci.2004;4(8):811-819.[14] He Q,Gong K,Ao Q,et al.Positive charge of chitosan retards blood coagulation on chitosan films.J Biomater Appl.2011 [Epub ahead of print].[15] Ho CH. The hemostatic effect of packed red cell transfusion in patients with anemia. Transfusion.1998;38(11-12):1011-1014. |