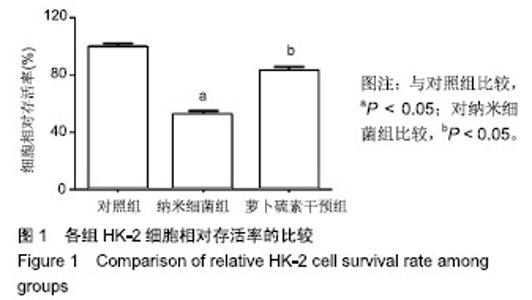
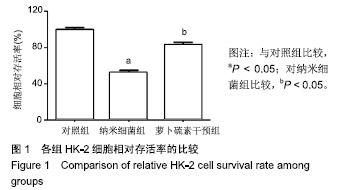
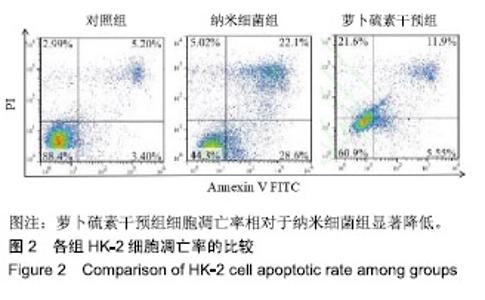
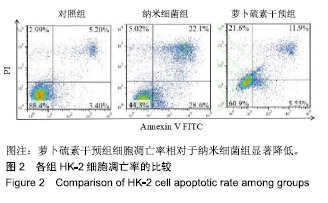
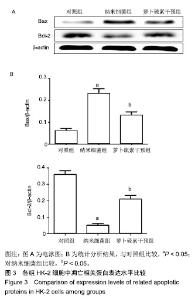
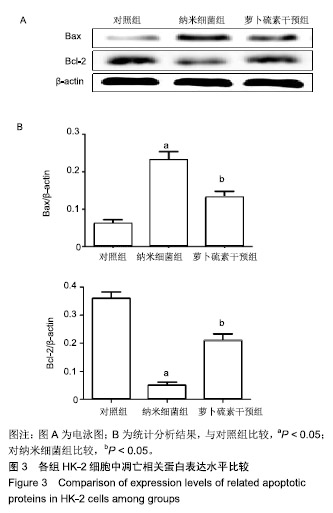
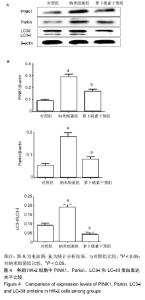
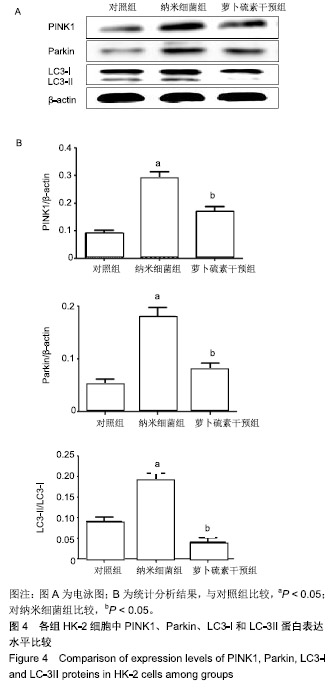
| [1]刘亚玮,李建坤,赵春艳,等.体外震波碎石术后应用中药排石汤治疗肾结石患者的疗效观察[J]. 中国药房, 2016,27(8): 1110-1112.[2]Abrol N, Panda A, Kekre NS, et al. Nanobacteria inthe pathogenesis of urolithiasis:myth or reality. IndianJ Urol. 2015;31(1): 3-7.[3]申茂磊,高存祥,郑丽英,等. 四环素对纳米细菌感染的人肾小管上皮细胞HK-2中CaSR?Claudin-14表达的影响[J]. 中国药房, 2018, 29(19): 2607-2611.[4]Tan AL, Sourris KC, Harcourt BE, et al. Disparate effects on renal and oxidative parameters following RAGE deletion, AGE accumulation inhibition, or dietary AGE control in experimental diabetic nephropathy. Am J Physiol Renal Physiol. 2010; 298(3): F763-771.[5]Zhao C, Chen Z, Qi J, et al. Drp1-dependent mitophagy protects against cisplatin-induced apoptosis of renal tubular epithelial cells by improving mitochondrial function. Oncotarget. 2017; 8(13):20988-21000. [6]Ding H, Bai F, Cao H, et al. PDE/cAMP/Epac/C/EBP-β signaling cascade regulates mitochondria biogenesis of tubularEpithelial cells in renal fibrosis. Antioxid Redox Signal. 2018; 29(7):637-652.[7]Milián L,Peris JE,Gandía P, et al. Tenofovir-induced toxicity in renal proximal tubular epithelial cells: involvement of mitochondria. AIDS. 2017; 31(12):1679-1684. [8]Fischer F,Hamann A,Osiewacz HD. Mitochondrialqualitycontrol: an integrated network of pathways. Trends Biochem Sci. 2012; 37(7): 284-291.[9]Tortorella SM,Royce SG,Licciardi PV, et al. Dietary sulforaphane in cancer chemoprevention: the role of epigenetic regulation and HDAC inhibition.Antioxid Redox Signal. 2015; 22(16):1382-1424.[10]Nallasamy P,Si H,Babu PV, et al.Sulforaphane reduces vascular inflammation in mice and prevents TNF-α-induced monocyte adhesion to primary endothelial cells through interfering with the NF-κB pathway.J Nutr Biochem.2014; 25(8):824-833.[11]Li B, Kim DS, Yadav RK, et al. Sulforaphane prevents doxorubicin-induced oxidative stress and cell death in rat H9c2 cells.Int J Mol Med.2015; 36(1):53-64.[12]Park JH, Kim JW, Lee CM, et al. Sulforaphane inhibits the Th2 immune response in ovalbumin-induced asthma.BMB Rep. 2012; 45(5):311-316.[13]Cekauskas A, Bruns H, Manikas M, et al. Sulforaphane decreases kidney injury after transplantation in rats: role of mitochondrial damage.Ann Transplant. 2013;18(2):488-496. [14]Juengel E, Maxeiner S, Rutz J, et al. Sulforaphane inhibits proliferation and invasive activity of everolimus-resistant kidney cancer cells in vitro.Oncotarget. 2016; 7(51): 85208-85219.[15]Çíftçíoglu N, Miller-Hjelle MA, Hjelle JT, et al. Inhibition of nanobacteria by antimicrobial drugs as measured by a modified microdilution method.Antimicrob Agents Chemother. 2002;46(7):2077-2086.[16]Hong X, Wang X, Wang T, et al. Role of nanobacteria in the pathogenesis of kidney stone formation.Am J Transl Res. 2016;8(7):3227-3234.[17]Yu CF, Huang XB, Chen L, et al.Effect of nanobacteria on cell damage and crystal retention in renal tubular epithelial cells. Beijing Da Xue Xue Bao Yi Xue Ban.2010; 42(4):436-442.[18]Czajka A, Ajaz S, Gnudi L, et al. Altered mitochondrial function, mitochondrial DNA and reduced metabolic flexibility in patients with diabetic nephropathy. EBioMedicine.2015;2(6): 499-504.[19]Sharma K, Karl B, Mathew AV, et al. Metabolomics reveals signature of mitochondrial dysfunction in diabetic kidney disease. J Am Soc Nephrol.2013; 24(11): 1901-1908.[20]Supale S, Li N, Brun T, et al. Mitochondrial dysfunction in pancreatic β cells. Trends Endocrin Met.2012;23(9): 477-482.[21]allabh NA,Romano V,Willoughby CE.Mitochondrial dysfunction and oxidative stress in corneal disease. Mitochondrion.2017; 36(12):103-113.[22]West AP.Mitochondrial dysfunction as a trigger of innate immune responses and inflammation.Toxicology.;2017; 391(25):54-63.[23]Bhargava P, Schnellmann RG.Mitochondrial energetics in the kidney.Nat Rev Nephrol.2017;13(10):629-646. [24]Eiyama A, Okamoto K. PINK1/Parkin-mediated mitophagy in mammalian cells. Curr Opin Cell Biol.2015;33(4): 95-103.[25]Gao W, Chen Z, Wang W, et al. E1-like activating enzyme Atg7 is preferentially sequestered into p62 aggregates via its interaction with LC3-I.PLoS One.2013; 8(9):e73229. [26]Pankiv S,Clausen TH,Lamark T, et al.p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy.J Biol Chem.2007; 282(33): 24131-24145.[27]Honda A, Komiya K, Hara A, et al. Normal pancreatic β-cell function in mice with RIP-Cre-mediated inactivation of p62/SQSTM1.Endocr J.2018; 65(1):83-89. [28]覃正碧,毛卫林. 萝卜硫素对肾小管上皮细胞氧化应激及Nrf2 / HO -1信号通路的影响[J]. 中国药师, 2017, 20(5): 809-811.[29]Nguyen B, Luong L, Naase H, et al. Sulforaphane pretreatment prevents systemic inflammation and renal injury in response to cardiopulmonary bypass.J Thorac Cardiovasc Surg.2014; 148(2):690-697. |