Chinese Journal of Tissue Engineering Research ›› 2021, Vol. 25 ›› Issue (6): 935-941.doi: 10.3969/j.issn.2095-4344.4001
Previous Articles Next Articles
Pathogenesis of hormonal osteonecrosis of the femoral head and the target effect of related signaling pathways
Li Shibin1, Lai Yu1, Zhou Yi1, Liao Jianzhao1, Zhang Xiaoyun1, Zhang Xuan2
- 1Department of Traumatology Orthopedics and Hand Surgery, Ruikang Hospital Affiliated to Guangxi University of Chinese Medicine, Nanning 530011, Guangxi Zhuang Autonomous Region, China; 2College of Orthopedic Injury, Guangxi University of Chinese Medicine, Nanning 530001, Guangxi Zhuang Autonomous Region, China
-
Received:2020-04-11Revised:2020-04-15Accepted:2020-05-13Online:2021-02-28Published:2020-12-04 -
Contact:Zhang Xuan, MD, Attending physician, College of Orthopedic Injury, Guangxi University of Chinese Medicine, Nanning 530001, Guangxi Zhuang Autonomous Region, China Zhang Xiaoyun, Attending physician, Doctoral candidate, Department of Traumatology Orthopedics and Hand Surgery, Ruikang Hospital Affiliated to Guangxi University of Chinese Medicine, Nanning 530011, Guangxi Zhuang Autonomous Region, China -
About author:Li Shibin, Master candidate, Department of Traumatology Orthopedics and Hand Surgery, Ruikang Hospital Affiliated to Guangxi University of Chinese Medicine, Nanning 530011, Guangxi Zhuang Autonomous Region, China -
Supported by:the National Natural Science Foundation of China, No. 81760796 and 81960803; the Basic Research Ability of Young and Middle-aged Teachers in Colleges and Universities of Guangxi Zhuang Autonomous Region of China, No. 2019KY0352; the Science and Research Project of Guangxi University of Chinese Medicine in 2019, No. 2019QN027; the First-Level Subject Project of Guangxi University of Chinese Medicine, No. 2019XK029; the Natural Science Foundation Project of Guangxi, No. 2019JJB140139; the PhD Research Startup Foundation of Guangxi University of Chinese Medicine in 2017, No. 2017BS038; the First-Level Subject Project of Guangxi University of Chinese Medicine, No. 2019XK027; the Chongzuo Municipal Science and Technology Plan Project of 2019, No. Chongke FC2019006
CLC Number:
Cite this article
Li Shibin, Lai Yu, Zhou Yi, Liao Jianzhao, Zhang Xiaoyun, Zhang Xuan. Pathogenesis of hormonal osteonecrosis of the femoral head and the target effect of related signaling pathways[J]. Chinese Journal of Tissue Engineering Research, 2021, 25(6): 935-941.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
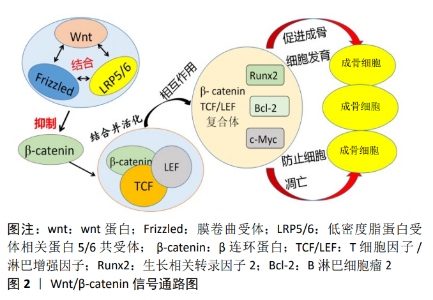
2.1 激素性股骨头坏死发病机制的研究 2.1.1 血管内凝血理论 非创伤性股骨头坏死最常见的病理生理改变是血管内凝血和微循环血栓形成。许多事例表明,股骨头缺血性坏死患者存在凝血和低纤溶的倾向[7],弥散性血管内凝血理论被认为是股骨头坏死的直接原因[8]。目前发现股骨头微循环的血栓闭塞与低纤维蛋白溶解(溶解血管内血栓的能力降低)和血栓性疾病有关,而血栓性疾病通常是由肝源性抗凝剂水平降低引起的,低纤维蛋白溶解可能是由于组织纤溶酶原激活物(tissue plasminogen activator,TPA)水平降低或纤溶酶原激活物抑制剂1 (PAI-1)水平升高所造成。 JONES等[9]研究了9种不同的凝血因子,发现有至少一种凝血因子异常的骨坏死患者高达82%,而对照组为30%;47%的患者有2种或2种以上凝血因子异常,而对照组为2.5%。VAROGA等[10]从股骨头缺血性坏死患者坏死组织周围的区域取得材料,经过免疫组织化学染色研究发现,坏死区域的边缘与其他区域相比显著增加了血管内皮生长因子(vascular endothelial growth factor,VEGF)的含量,并且发现骨坏死可能刺激小动脉修复性向内生长进入股骨头。LI等[11]在动物实验中发现去铁胺通过改善缺氧诱导因子1α(hypoxia inducible factor-1,HIF-1α)、血管内皮生长因子、骨形态发生蛋白2(bone morphogenetic protein-2,BMP-2)和骨钙素(osteocalcin,OCN)的表达,诱导股骨头血管生成和改善骨质量来提高坏死股骨头的骨修复能力。总之目前在对股骨头坏死的病理检查中发现,大部分激素性股骨头坏死患者存在血管内血栓形成,这进一步为血管内凝血理论提供了有力的证据支撑。 2.1.2 骨髓间充质干细胞分化理论 骨髓间充质干细胞是一种能够分化成多种骨基质细胞的多能干细胞,其成骨分化与成脂分化之间的不平衡被认为是激素性股骨头坏死发病的主要机制。目前发现类固醇激素除了增强脂肪细胞特异性基因的表达外,还降低了Ⅰ型胶原及骨钙素mRNA的表达,骨髓遭脂质浸润后胶原和骨钙素都减少,加快了可用于修复损伤的骨祖细胞死亡。据实验报道,高浓度地塞米松可通过抑制骨髓间充质干细胞的功能,从而导致成骨分化和成脂分化失衡,减少骨形成[12]。另外在对激素性股骨头坏死兔模型的观察中发现,其骨髓造血和基质细胞的活性水平、间充质细胞的数量均降低,长期类固醇治疗刺激多能骨髓基质细胞分化为脂肪细胞[13]。骨干细胞中类固醇诱导的脂肪生成和脂质代谢的系统性变化是类固醇诱导的骨质疏松症和骨坏死的主要原因,脂肪细胞肥大可导致骨内压升高,产生骨内分隔综合征;黏附在血管内壁上的脂肪团块形成脂肪栓子,骨髓细胞此时被脂肪细胞占据,脂肪细胞融合成碎片导致骨髓中的造血细胞死亡,最终造成缺血和骨坏死。Houdek等[14]发现与从皮质类固醇激素诱导的骨坏死患者中分离的骨髓间充质干细胞相比,股骨颈骨折患者的骨髓间充质干细胞显示出更大的细胞生长潜能和更强的骨分化能力,这就进一步证明了类固醇激素降低骨髓间充质干细胞的成骨潜能,增加其成脂分化。 2.1.3 脂质代谢紊乱理论 脂代谢异常是非创伤性股骨头坏死的一个重要危险因素。目前发现激素能诱导脂质代谢发生紊乱,从而导致微血管脂肪栓塞和骨细胞脂肪沉积,并最终引起股骨头缺血性坏死。高剂量使用激素会使患者皮下脂肪动员显著增加,随着给药时间的延长,会引起血脂增高并导致高脂血症,使体内血液趋于停滞,此时全身脂肪栓塞情况严重,股骨头微小颗粒形成脂肪栓直接导致局部血瘀,骨髓细胞被大量脂肪细胞所占据,脂肪细胞融合成碎片造成骨髓衍生细胞死亡,最终导致股骨头缺血性坏死[15]。 KURODA等[16]发现在需要大剂量泼尼松龙治疗的系统性红斑狼疮患者中,高三酰甘油水平是诱导激素性股骨头坏死发生的重要危险因素。此外ZHANG等[17]通过对 223例非创伤性股骨头坏死患者进行分组研究,发现大多数激素性股骨头坏死患者血液生化指标异常,尤其是血脂水平,并提出血脂检查可用作高危人群中非创伤性股骨头坏死、乙醇中毒和滥用激素的筛查工具。滥用激素会导致患者脂代谢紊乱、血液黏稠、骨细胞脂肪蓄积及脂肪变性,这些变化最终会导致营养骨细胞的血流量减少、血液凝固,加重骨髓腔受到压迫,股骨头中的小血管被减少或阻塞,最终导致缺血性骨坏死。NOZAKI等[18]在动物实验中发现普伐他汀可通过抑制过氧化物酶体增殖物激活受体γ(peroxisome proliferators-activated receptors-γ,PPAR-γ)的表达及在mRNA和蛋白水平上增加Wnt3a、低密度脂蛋白受体相关蛋白5(low density lipoprotein receptor-related protein,LRP5)、β-连环蛋白和生长相关转录因子2 (runt-related transcription factor 2,RUNX2)的表达来积极调节脂质代谢,从而有效预防了激素诱导的股骨头坏死。JIANG等[19]通过试验证实中药HuoguⅠ号配方具有降低血脂、抑制过氧化物酶体增殖物激活受体-γ基因表达、激活Wnt/LRP5/β-catenin信号通路并促进成骨基因表达等作用,通过抑制脂肪形成相关基因表达最终防止激素性股骨头坏死的发生。 2.1.4 骨质疏松理论 骨质疏松是长期不当使用糖皮质激素所造成的主要负面影响,被认为是引起激素性股骨头坏死的发病机制之一。糖皮质激素能够大量消耗蛋白质并使其合成减低,同时对抗维生素D并使胃肠道对钙吸收产生障碍,从而造成大量钙被排泄[20]。另一方面大剂量应用糖皮质激素可降低成骨细胞的活性并抑制破骨细胞骨吸收,减少骨转换[21],通过激活或抑制成骨和成脂相关转录调控因子,影响间充质干细胞的分化,最终使间充质干细胞来源的脂肪细胞的大小和数量显著增加,造成成骨细胞数量显著减少,此时骨小梁严重稀疏引起骨质疏松,最终导致股骨头承重区塌陷,甚至坏死[22]。 刘慧等[23]通过基因芯片技术对股骨头坏死进行研究,发现糖皮质激素可以减少骨代谢相关基因的表达从而对骨重塑及软骨代谢造成破坏,并最终引起骨质疏松的发生。生长相关转录因子2是成骨细胞形成的关键转录调控因子,在成骨细胞成熟和稳态中起着基础性作用,通过多种途径调节骨髓间充质干细胞的分化。在研究中发现生长相关转录因子2同样受到糖皮质激素的调节,当对必须接受类固醇药物治疗的患者给予高剂量的糖皮质激素时,生长相关转录因子2的水平将显著降低,而Runx2的表达下调,将导致骨髓间充质干细胞衍生的骨脂肪细胞增多,而成骨细胞数量显著减少,最终导致骨内的压力增高和股骨头血流量减少[24]。另外在对小鼠的实验中表明大量使用糖皮质激素,会造成小鼠股骨头部位的骨量明显减少及骨质变脆,时间过长导致股骨头严重塌陷及坏死[25]。 2.1.5 骨细胞凋亡理论 随着对激素诱导的股骨头坏死发病机制的深入研究,骨细胞凋亡理论越来越受到关注,大量研究表明激素性股骨头坏死是骨细胞大量凋亡的表现。患者在长期服用糖皮质激素后,骨细胞的机械感受功能遭到破坏,股骨皮质成骨细胞和骨细胞大多发生凋亡[26],骨细胞的凋亡会导致骨小梁变得稀疏,骨质减少,此时经受轻微的外力便容易致使股骨头部位发生折断,进一步引起股骨头发生坍塌,最后股骨头缺血性坏死形成[27]。 有研究通过实验在光学显微镜下观察糖皮质激素诱导的兔股骨头坏死模型的染色切片[28],发现股骨头软骨下骨的骨小梁变薄,骨细胞凋亡在骨小梁周围特殊染色中大量存在。此外在动物模型实验中发现左旋多巴能够促进体内代谢中胰岛素样生长因子1(insulin-like growth factor 1,IGF-1)的合成和释放,有效减少了类固醇诱导的骨细胞凋亡和股骨头坏死的发生,是预防和治疗股骨头坏死的可靠途径[29]。CHEN等[30]也在实验中发现促红细胞生成素通过抑制成骨细胞和骨细胞的凋亡及增加血管内皮生长因子的表达,对糖皮质激素诱导的大鼠股骨头坏死具有显著的保护作用。ZHANG等[31]对兔使用地塞米松进行股骨头缺血性坏死造模实验,分别在造模前后对兔的细胞凋亡及 Bcl-2、Caspase-8等表达水平进行检测,发现激素能够影响Bcl-2的表达并激活Caspase-8,以此引起骨细胞发生凋亡。此外有研究证实了阿魏酸钠通过降低caspase-3的表达和上调Bcl-2的表达,从而有效干预细胞凋亡,显著降低兔早期激素性股骨头坏死的发生率[32]。 总之近些年大量研究者通过实验证明了一个结论:激素的不合理使用能够使相关基因表达发生障碍从而造成骨细胞凋亡,并最终导致股骨头坏死的发生[33]。 2.2 激素性股骨头坏死在细胞通路的研究 2.2.1 Wnt信号通路 Wnts是一个进化上保守的生长因子家族,其信号通路参与了发育、成体和疾病发生的多种过程[34]。目前发现Wnt/β-catenin及DKK-1信号通路在维持健康的骨量方面发挥着重要的作用。 (1) β-catenin:Wnt/β-catenin 信号通路作为Wnt信号通路转导中的经典通路,目前已经被证实了在骨形成过程中起着关键作用。它通过多种机制如促进干细胞的更新、成骨细胞的增殖、诱导成骨细胞形成以及抑制成骨细胞和骨细胞凋亡等促进骨量的增加[35]。 在通路形成中,wnt蛋白首先与膜卷曲受体(Frizzled)和LRP5/6 共受体相互结合,破坏细胞内 β-catenin的活性,并引导其进入核内。接下来核内的β-catenin和T细胞因子/淋巴增强因子相互作用,其合成体与生长相关转录因子2、Bcl-2、c-Myc等基因结合,从而激活下游的信号,发挥刺激骨细胞的发育及防止细胞凋亡的调控作用[36-37],见图2。同时Wnt/β-catenin 信号通路是调控髓基质细胞表达的关 键[38]。如前所述,许多因素通过影响骨髓间充质干细胞的分化而导致股骨头坏死,生长相关转录因子2、DKK-1和过氧化物酶体增殖物激活受体γ则被认为是分化过程中最重要的因素,而Wnt/β-catenin 信号通路通过其下游信号过氧化物酶体增殖物激活受体γ能够抑制骨髓间充质干细胞的正常分化[39]。过氧化物酶体增殖物激活受体γ作为脂肪生成的主要调节因子,大量使用糖皮质激素可以使其表达增加,诱导脂肪细胞分化并加速骨髓间充质干细胞向脂肪细胞系的转移,由此使间充质细胞中成骨细胞分化产生障碍,引起成熟的成骨细胞存活率较低,此时骨脂肪细胞大量生成,骨髓间充质干细胞向成骨和成脂细胞分化的稳态产生了显著异常变化,最终导致骨内压升高和股骨头缺血[40-41]。而在最近的实验中有力地证实了这一点,Wnt/β-catenin 信号通路可以在锂剂的作用下对骨髓间充质干细胞的分化产生明显的影响,其降低了成脂细胞的分化,增强了成骨作用及骨吸收,最终加快了骨再生[42]。 "

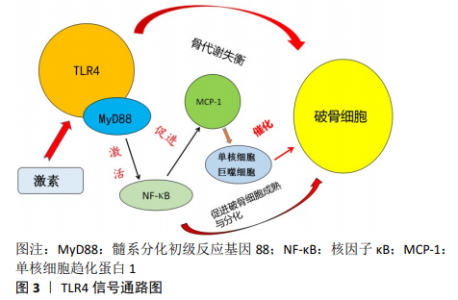
(2) DKK-1:DKK是Wnt家族的成员,DKK家族由脊椎动物中的4个主要成员(DKK-1,2,3,4)组成,DKK-1是非洲爪蟾胚胎头部诱导因子和Wnt拮抗剂,大量证据支持DKK-1在Wnt/β-catenin信号转导中的作用,DKK-1的过度表达将引起细胞凋亡和生长抑制。目前发现细胞因子、巨噬细胞集落刺激因子(macrophage colony-stimulating factor,M-CSF)、核因子κB受体激活剂配体(receptor activator of nuclear factor-κB ligand,RANKL)和DKK-1对破骨细胞的发育至关重要,在正常健康的骨中,骨形成和吸收的平衡在很大程度上是通过这些细胞类型与其干细胞前体的协同分化来实现的。 糖皮质激素的使用不仅增加了DKK-1和分泌卷曲相关蛋白1(secreted frizzled-related protein 1,SFRP-1)的表达,通过激活DKK-1和延长破骨细胞的寿命来影响破骨细胞的活性从而增强骨吸收,并且抑制Wnt/β-catenin信号传导[43],最终抑制骨再生。目前经过了充足的研究,证实了在激素性股骨头坏死的进程中,不当使用激素会增强DKK-1的表达,抑制了间充质细胞向成骨细胞的分化,成骨细胞的数量减少,加快骨细胞发生凋亡,造成骨形成及再生能力降低,最终骨量减少引起股骨头塌陷和坏死[44]。 综上,充分理解Wnt信号通路在激素性股骨头坏死中的相关作用机制对于研究人类骨坏死的是非常重要的,临床值得进一步探讨。 2.2.2 TLR4信号通路 Toll样受体4(Toll-like Receptors4,TLR4)是Toll样受体家族中最常见的成员之一,作为一种主要在单核细胞/巨噬细胞和树突细胞中表达的模式识别受体,其与病原体相关的分子模式结合,参与先天免疫和获得性免疫应答[45]。同时它也在骨细胞中表达,其激活有助于TLR配体诱导的破骨细胞生成并影响破骨细胞分化。 目前发现TLR4通路的信号转导主要依赖于髓系分化初级反应基因88(myeloiddifferentiationfactor88,MyD88)。髓系分化初级反应基因88能介导信号传导至下游,并且能激活核因子κB。核因子κB目前被认为是最重要的转录因子之一,其调节多种炎症和免疫基因的表达,它参与下游炎症通路和TLR4信号通路,诱导共刺激分子表达以促进破骨细胞前体细胞的分化并提高成熟破骨细胞的存活率[46]。另外核因子κB还可激活单核细胞趋化蛋白1(Monocyte Chemotactic Protein 1,MCP-1),诱导单核细胞/巨噬细胞增殖,最终形成破骨细胞,见图3。TIAN等[47]在动物股骨头坏死模型中发现,糖皮质激素的大量使用可诱导TLR4信号通路高度表达,并使免疫反应发生异常,同时核因子κB被活化,打破了骨代谢的平衡,大量破骨细胞被激活和增殖,从而出现股骨头坏死。另外在最近的研究中发现,TLR4在抑制Wnt/β-catenin信号通路的情况下也可以使激素性股骨头坏死模型的骨骼生成障碍,而在使用核因子κB抑制剂后,Wnt/β-catenin信号通路则会发生强表达,股骨头坏死率大幅度减低[48]。 目前许多研究证实了TLR4信号通路在类固醇(糖皮质激素)诱导的股骨头坏死的发病机制中起着至关重要的作用。针对TLR4的研究,防止骨坏死发生的关键策略之一是减少破骨细胞前体细胞的生成。临床上的治疗策略可以往免疫调节生物药物,特别是作用于效应分子的药物方面展开,可能在股骨头坏死的治疗中有良好的效果。"

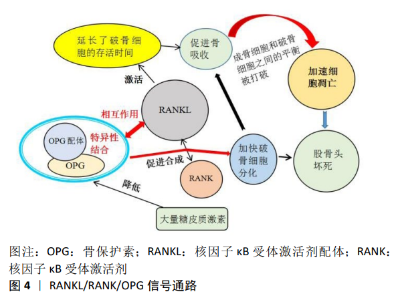
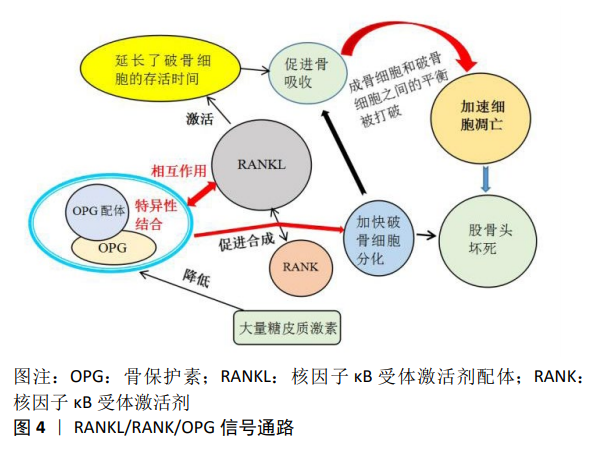
2.2.3 RANKL/RANK/OPG信号通路 RANKL/RANK/OPG信号通路主要由核因子κB受体激活剂配体,核因子κB受体激活剂(receptor activator of nuclear factor-κB,RANK),骨保护素(osteoprotegerin,OPG),肿瘤坏死因子受体相关因子6 (TNF receptor associated factor,TRAF6),和活化T-细胞核因子1 (nuclear factor of activated T-cells cytoplasmic 1,NFATC1)等5种蛋白质组成。该途径在调节骨重塑、破骨细胞分化和多种病理状态中发挥关键的作用,目前因其能介导成骨细胞和破骨细胞之间的平衡以及骨髓间充质干细胞中成骨和成脂的异常转分化,被认为在股骨头坏死的分子机制中起中心作用[49]。 破骨细胞的存活和分化受基质细胞和成骨细胞产生的因子调控,其关键因子是肿瘤坏死因子(tumor necrosis factor,TNF)配体家族成员之一的核因子κB受体激活剂配体。在没有骨髓基质细胞的情况下,核因子κB受体激活剂配体和巨噬细胞集落刺激因子对于破骨细胞分化是至关重要的,其延长了分化的破骨细胞的存活时间,通过信号级联反应激活破骨细胞以促进骨吸收,致使成骨细胞和破骨细胞之间的平衡被打破,局部骨代谢的稳定性随之遭到破坏,加速细胞凋亡[50],见图4。骨保护素是由肿瘤坏死因子超家族11b(TNFRSF11B)编码的蛋白,是一种成骨细胞分泌的诱饵受体,作为骨吸收的负性调节因子,它与骨保护素配体特异性结合,是破骨细胞发育的关键细胞外调节因子[51]。研究发现骨保护素通过与破骨细胞表皮的核因子κB受体激活剂配体发生作用从而阻止RANK与核因子κB受体激活剂配体的合成,发挥阻断破骨细胞的分化,维持骨稳态的功能[52]。 以往研究报道,大量使用糖皮质激素延长了破骨细胞的寿命,将直接导致早期骨丢失的发生。另外SCHAFFLER等[53]的研究也证明了使用糖皮质激素会导致股骨头部位骨保护素的表达水平显著下降,破骨细胞得以快速分化、成熟,促进了骨吸收能力,骨质得到大量丢失,骨小梁结构遭到破坏,最终引起股骨头坏死。 2.2.4 TGF-βSmad信号通路 TGF-βSmad是目前备受关注的一条信号通路,其在细胞增殖、分化和迁移等过程中发挥着关键的作用。转化生长因子β(transforming growth factor-β,TGF-β)超家族由40多个成员组成,如转化生长因子β、活化素、抑制素、骨形态发生蛋白、生长分化因子(growth differentiation factors,GDF)等。转化生长因子β1是人体骨骼中含量最丰富的生长因子,通过促进间充质细胞的增殖分化、成骨细胞的增殖分化和骨基质的生成,在促进骨形成中发挥重要作用。Smad蛋白被认为是转化生长因子-β1的主要细胞内信号转导分子。转化生长因子β1启动Smads信号通路以调节成骨细胞功能,从而磷酸化smad2和smad3,然后磷酸化的smad2/3与smad4结合形成三聚体复合物,然后转运到细胞核中以启动靶基因的转录并刺激骨形成[54]。 研究结果表明,转化生长因子β超家族成员通过异聚体受体复合物发挥作用,该复合物由细胞表面的Ⅰ型和Ⅱ型受体组成,通过Smad复合物或丝裂原活化蛋白激酶 (mitogen-activated protein kinase,MAPK)级联传导细胞内信号[55]。目前转化生长因子β Smad信号通路被认为与激素性股骨头坏死的发生紧密相关。糖皮质激素因其抗炎及免疫抑制功能造成转化生长因子β/Smad的活性下降,导致成骨细胞增殖障碍,骨基质的生成减少,同时引起股骨头血管内皮细胞发育遭到破坏,血管成熟延缓,血流量不足最终造成股骨头坏死的发生[56]。YANG等[57]在动物造模实验中发现,淫羊藿和女贞子能够增强转化生长因子β/Smad信号通路的活性,加快成骨细胞的增殖及分化,在促进骨形成中发挥重要作用,有效降低了股骨头坏死的发生。"
| [1] ZHANG Y, LIU Y, ZHOU G, et al. Fibular allograft for osteonecrosis prevention in the management of femoral neck fractures: clinical outcome and biomechanical evaluation. J Biomater Tissue Eng. 2015; 5(12):937-941. [2] 王耀霆,梁豪君,姜川,等. MRI指导下经皮钻孔减压术治疗股骨头缺血性坏死的疗效[J].武警医学,2020,31(2):132-135. [3] WIN AZ, APARICI CM. Non-traumatic radiation-induced avascular necrosis of the femoral neck. QJM. 2015;108(3):257-258. [4] GÓMEZ-MONT LANDERRECHE JG, GIL-ORBEZO F, MORALES-DOMINGUEZ H, et al. Nontraumatic causes of bilateral avascular necrosis of the femoral head: link between hepatitis C and pegylated interferon. Acta Ortop Mex. 2015;29(3):172-175. [5] CHAO PC, CUI MY, LI XA, et al. Correlation between miR-1207-5p expression with steroid-induced necrosis of femoral head and VEGF expression. Eur Rev Med Pharmacol Sci. 2019;23(7):2710-2718. [6] LI GQ, WANG ZY. MiR-20b promotes osteocyte apoptosis in rats with steroid-induced necrosis of the femoral head through BMP signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23(11):4599-4608. [7] FANG SH, LI YF, JIANG JR, et al. Relationship of α2-Macroglobulin with steroid-induced femoral head necrosis: a Chinese population-based association study in southeast China. J Orthop Surg. 2019;11(3): 481-486. [8] WANG A, REN M, WANG J. The pathogenesis of steroid-induced osteonecrosis of the femoral head: a systematic review of the literature. Gene. 2018;671:103-109. [9] JONES LC, HUNGERFORD DS. Osteonecrosis: etiology, diagnosis, and treatment. Curr Opin Rheumatol. 2004;16(4):443-449. [10] VAROGA D, DRESCHER W, PUFE M, et al. Differential expression of vascular endothelial growth factor in glucocorticoid-related osteonecrosis of the femoral head. Clin Orthop Relat Res. 2009;467(12): 3273-3282. [11] LI J, FAN L, YU Z, et al. The effect of deferoxamine on angiogenesis and bone repair in steroid-induced osteonecrosis of rabbit femoral heads. Exp Biol Med (Maywood). 2015;240(2):273-280. [12] ZHAO SR, WEN JJ, MU HB. Role of Hsa-miR-122-3p in steroid-induced necrosis of femoral head. Eur Rev Med Pharmacol Sci. 2019;23 (3 Suppl): 54-59. [13] ZHANG P, TAO F, LI Q, et al. 5-Azacytidine and trichostatin A enhance the osteogenic differentiation of bone marrow mesenchymal stem cells isolated from steroid-induced avascular necrosis of the femoral head in rabbit. J Biosci. 2019;44(4):87. [14] HOUDEK MT, WYLES CC, PACKARD BD, et al. Decreased Osteogenic Activity of Mesenchymal Stem Cells in Patients With Corticosteroid-Induced Osteonecrosis of the Femoral Head. J Arthroplasty. 2016; 31(4):893-898. [15] REN X, FAN W, SHAO Z, et al. A metabolomic study on early detection of steroid-induced avascular necrosis of the femoral head. Oncotarget. 2018;9(8):7984-7995. [16] KURODA T, TANABE N, WAKAMATSU A, et al. High triglyceride is a risk factor for silent osteonecrosis of the femoral head in systemic lupus erythematosus. Clin Rheumatol. 2015;34(12):2071-2077. [17] ZHANG Y, SUN R, ZHANG L, et al. Effect of blood biochemical factors on nontraumatic necrosis of the femoral head: Logistic regression analysis. Orthopade. 2017;46(9):737-743. [18] NOZAKI Y, KUMAGAI K, MIYATA N, et al. Pravastatin reduces steroid-induced osteonecrosis of the femoral head in SHRSP rats. Acta Orthop. 2012;83(1):87-92. [19] Jiang Y, Liu D, Kong X, et al. Huogu I formula prevents steroid-induced osteonecrosis in rats by down-regulating PPARgamma expression and activating wnt/LRP5/ beta-catenin signaling. J Tradit Chin Med. 2014;34(3):342-350. [20] Li H, Meng D, Zhang X, et al. Effect of psoralen on the expression of PPARγ, osteocalcin, and trabecular bone area in rabbits with steroid-induced avascular necrosis of the femoral head. J Orthop Surg Res. 2019;14(1):11. [21] 高彦淳,冯勇,张长青.激素性股骨头坏死发生机制的研究进展[J].国际骨科学杂志,2018,39(4):231-234. [22] 康少平,刘淑艳,李永升,等.激素性股骨头坏死患者骨髓间充质干细胞体外增殖分化能力的研究[J].中国细胞生物学学报,2015, 37(11): 1490-1496. [23] 刘慧,许冰,程婉,等.大鼠激素性股骨头坏死骨代谢差异表达基因的实验研究[J].河南中医,2011,31(8):856-860. [24] TAN G, KANG PD, PEI FX. Glucocorticoids affect the metabolism of bone marrow stromal cells and lead to osteonecrosis of the femoral head: a review. Chin Med J (Engl). 2012;125(1):134-139. [25] WEINSTEIN RS, HOGAN EA, BORRELLI MJ, et al. The Pathophysiological Sequence of Glucocorticoid-Induced Osteonecrosis of the Femoral Head in Male Mice. Endocrinology. 2017;158(11):3817-3831. [26] LUO H, LAN W, LI Y, et al. Microarray analysis of long-noncoding RNAs and mRNA expression profiles in human steroid-induced avascular necrosis of the femoral head. J Cell Biochem. 2019;120(9):15800-15813. [27] 芮仞,娄悦,张先姚,等.细胞凋亡在激素性股骨头坏死发病机制中的研究进展[J].山西中医学院学报,2019, 20(1):65-69. [28] LIVAK KJ, SCHMITTGEN TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402-408. [29] XI H, TAO W, JIAN Z, et al. Levodopa attenuates cellular apoptosis in steroid-associated necrosis of the femoral head. Exp Ther Med. 2017; 13(1):69-74. [30] CHEN S, LI J, PENG H, et al. Administration of erythropoietin exerts protective effects against glucocorticoid-induced osteonecrosis of the femoral head in rats. Int J Mol Med. 2014;33(4):840-848. [31] ZHANG H, ZHOU F, PAN Z, et al. 11β-hydroxysteroid dehydrogenases-2 decreases the apoptosis of MC3T3/MLO-Y4 cells induced by glucocorticoids. Biochem Biophys Res Commun. 2017;490(4): 1399-1406. [32] TIAN L, DANG XQ, WANG CS, et al. Effects of sodium ferulate on preventing steroid-induced femoral head osteonecrosis in rabbits. J Zhejiang Univ Sci B. 2013;14(5):426-437. [33] LIU G, LUO G, BO Z, et al. Impaired osteogenic differentiation associated with connexin43/microRNA-206 in steroid-induced avascular necrosis of the femoral head. Exp Mol Pathol. 2016;101(1):89-99. [34] MOHAMMED MK, SHAO C, WANG J, et al. Wnt/β-catenin signaling plays an ever-expanding role in stem cell self-renewal, tumorigenesis and cancer chemoresistance. Genes Dis. 2016;3(1):11-40. [35] PEI J, FAN L, NAN K, et al. Excessive Activation of TLR4/NF-κB Interactively Suppresses the Canonical Wnt/β-catenin Pathway and Induces SANFH in SD Rats. Sci Rep. 2017;7(1):11928. [36] YAO Q, YU C, ZHANG X, et al. Wnt/β-catenin signaling in osteoblasts regulates global energy metabolism. Bone. 2017;97:175-183. [37] ZHANG C, ZOU YL, MA J, et al. Apoptosis associated with Wnt/β-catenin pathway leads to steroid-induced avascular necrosis of femoral head. BMC Musculoskelet Disord. 2015;16(1):132. [38] JIA J, FENG X, XU W, et al. MiR-17-5p modulates osteoblastic differentiation and cell proliferation by targeting SMAD7 in non-traumatic osteonecrosis. Exp Mol Med. 2014;46(7):e107. [39] WANG XL, WANG N, ZHENG LZ, et al. Phytoestrogenic molecule desmethylicaritin suppressed adipogenesis via Wnt/β-catenin signaling pathway. Eur J Pharmacol. 2013;714(1-3):254-260. [40] YUAN N, LI J, LI M, et al. BADGE, a synthetic antagonist for PPARγ, prevents steroid-related osteonecrosis in a rabbit model. BMC Musculoskelet Disord. 2018;19(1):129. [41] YU Z, FAN L, LI J, et al. Lithium prevents rat steroid-related osteonecrosis of the femoral head by β-catenin activation. Endocrine. 2016;52(2):380-390. [42] 翁诗阳,朱振中,汤春,等.锂介导的Wnt /β-catenin 信号转导通路对骨髓间充质干细胞的影响[J].国际骨科学杂志,2016,37(3): 183-186. [43] ZHANG F, CAO K, DU G, et al. miR-29a promotes osteoblast proliferation by downregulating DKK-1 expression and activating Wnt/β-catenin signaling pathway. Adv Clin Exp Med. 2019;28(10):1293-1300. [44] ZHUN W, DONGHAI L, ZHOUYUAN Y, et al. Efficiency of Cell Therapy to GC-Induced ONFH: BMSCs with Dkk-1 Interference Is Not Superior to Unmodified BMSCs. Stem Cells Int. 2018;2018:1340252. [45] 付志斌,李盛华,周明旺,等.非创伤性股骨头坏死相关信号通路研究进展[J].中国骨质疏松杂志,2017,23(4):555-560. [46] TIAN L, ZHOU DS, WANG KZ, et al. Association of toll-like receptor 4 signaling pathway with steroid-induced femoral head osteonecrosis in rats. J Huazhong Univ Sci Technolog Med Sci. 2014;34(5):679-686. [47] TIAN L, WEN Q, DANG X, et al. Immune response associated with Toll-like receptor 4 signaling pathway leads to steroid-induced femoral head osteonecrosis. BMC Musculoskelet Disord. 2014;15:18. [48] 朱道宇,杨前昊,高悠水,等.TLR4 通路与激素性股骨头坏死关系的研究进展[J].中国骨与关节杂志,2019,8(1):75-79. [49] MONT MA, CHERIAN JJ, SIERRA RJ, et al. Nontraumatic Osteonecrosis of the Femoral Head: Where Do We Stand Today? A Ten-Year Update. J Bone Joint Surg Am. 2015;97(19):1604-1627. [50] ZHOU L, LI L, WANG Y, et al. Effects of RANKL on the proliferation and apoptosis of fibroblast-like synoviocytes in rheumatoid arthritis through regulating the NF-κB signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23(21):9215-9221. [51] SONG Y, DU ZW, YANG QW, et al. Association of Genes Variants in RANKL/RANK/OPG Signaling Pathway with the Development of Osteonecrosis of the Femoral Head in Chinese Population. Int J Med Sci. 2017;14(7):690-697. [52] 黎彦龙,何明,陈秉雄,等.OPG-RANKL-RANK信号系统是调节破骨细胞及骨质疏松症的重要途径[J].中国组织工程研究, 2015, 19(24):3894-3898. [53] SCHAFFLER MB, KENNEDY OD. Osteocyte signaling in bone. Curr Osteoporos Rep. 2012;10(2):118-125. [54] MU Y, GUDEY SK, LANDSTRÖM M. Non-Smad signaling pathways. Cell Tissue Res. 2012;347(1):11-20. [55] WU M, CHEN G, LI YP. TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016;4:16009. [56] 卢非凡,张启栋,王卫国,等.激素性股骨头坏死信号通路的研究进展[J].中国矫形外科杂志,2018, 26(11):1017-1021. [57] YANG Y, NIAN H, TANG X, et al. Effects of the combined Herba Epimedii and Fructus Ligustri Lucidi on bone turnover and TGF-β1/Smads pathway in GIOP rats. J Ethnopharmacol. 2017;201:91-99. |
| [1] | Pu Rui, Chen Ziyang, Yuan Lingyan. Characteristics and effects of exosomes from different cell sources in cardioprotection [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 1-. |
| [2] | Xu Feng, Kang Hui, Wei Tanjun, Xi Jintao. Biomechanical analysis of different fixation methods of pedicle screws for thoracolumbar fracture [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1313-1317. |
| [3] | Jiang Yong, Luo Yi, Ding Yongli, Zhou Yong, Min Li, Tang Fan, Zhang Wenli, Duan Hong, Tu Chongqi. Von Mises stress on the influence of pelvic stability by precise sacral resection and clinical validation [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1318-1323. |
| [4] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [5] | Zhang Yu, Tian Shaoqi, Zeng Guobo, Hu Chuan. Risk factors for myocardial infarction following primary total joint arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1340-1345. |
| [6] | Wei Wei, Li Jian, Huang Linhai, Lan Mindong, Lu Xianwei, Huang Shaodong. Factors affecting fall fear in the first movement of elderly patients after total knee or hip arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1351-1355. |
| [7] | Wang Jinjun, Deng Zengfa, Liu Kang, He Zhiyong, Yu Xinping, Liang Jianji, Li Chen, Guo Zhouyang. Hemostatic effect and safety of intravenous drip of tranexamic acid combined with topical application of cocktail containing tranexamic acid in total knee arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1356-1361. |
| [8] | Xiao Guoqing, Liu Xuanze, Yan Yuhao, Zhong Xihong. Influencing factors of knee flexion limitation after total knee arthroplasty with posterior stabilized prostheses [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1362-1367. |
| [9] | Huang Zexiao, Yang Mei, Lin Shiwei, He Heyu. Correlation between the level of serum n-3 polyunsaturated fatty acids and quadriceps weakness in the early stage after total knee arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1375-1380. |
| [10] | Zhang Chong, Liu Zhiang, Yao Shuaihui, Gao Junsheng, Jiang Yan, Zhang Lu. Safety and effectiveness of topical application of tranexamic acid to reduce drainage of elderly femoral neck fractures after total hip arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1381-1386. |
| [11] | Wang Haiying, Lü Bing, Li Hui, Wang Shunyi. Posterior lumbar interbody fusion for degenerative lumbar spondylolisthesis: prediction of functional prognosis of patients based on spinopelvic parameters [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1393-1397. |
| [12] | Lü Zhen, Bai Jinzhu. A prospective study on the application of staged lumbar motion chain rehabilitation based on McKenzie’s technique after lumbar percutaneous transforaminal endoscopic discectomy [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1398-1403. |
| [13] | Chen Xinmin, Li Wenbiao, Xiong Kaikai, Xiong Xiaoyan, Zheng Liqin, Li Musheng, Zheng Yongze, Lin Ziling. Type A3.3 femoral intertrochanteric fracture with augmented proximal femoral nail anti-rotation in the elderly: finite element analysis of the optimal amount of bone cement [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1404-1409. |
| [14] | Du Xiupeng, Yang Zhaohui. Effect of degree of initial deformity of impacted femoral neck fractures under 65 years of age on femoral neck shortening [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1410-1416. |
| [15] | Zhang Shangpu, Ju Xiaodong, Song Hengyi, Dong Zhi, Wang Chen, Sun Guodong. Arthroscopic suture bridge technique with suture anchor in the treatment of acromioclavicular dislocation [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1417-1422. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||