Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (7): 1112-1117.doi: 10.12307/2024.102
Previous Articles Next Articles
Role of autophagy in hair regeneration
Huang Yuxin1, Liang Wenzi1, Chen Xiuwen1, Ni Na1, Zhao Yinglin2, Lin Changmin1
- 1Medical College, Shantou University, Shantou 515041, Guangdong Province, China; 2Mental Health Center, Shantou University, Shantou 515065, Guangdong Province, China
-
Received:2022-12-07Accepted:2023-03-06Online:2024-03-08Published:2023-07-17 -
Contact:Lin Changmin, Professor, Doctoral supervisor, Medical College, Shantou University, Shantou 515041, Guangdong Province, China -
About author:Huang Yuxin, Master candidate, Medical College, Shantou University, Shantou 515041, Guangdong Province, China -
Supported by:National Natural Science Foundation of China, No. 007-41369023 (to LCM)
CLC Number:
Cite this article
Huang Yuxin, Liang Wenzi, Chen Xiuwen, Ni Na, Zhao Yinglin, Lin Changmin. Role of autophagy in hair regeneration[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1112-1117.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
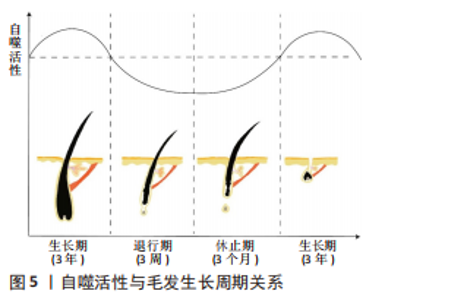
2.1 自噬活性与毛发生长周期的关系 毛囊是哺乳动物唯一终生呈现出周期性循环生长的器官[7]。人类毛发的生长周期可以分为生长期 (anagen)、退行期(catagen)和休止期(telogen),分别为3年、3周和3个月,其独特的周期性生长循环与毛囊中形态和功能各异的细胞间相互作用是密不可分的[8],其中毛乳头细胞和毛囊干细胞(hair follicle stem cells,HFSCs)对毛发生长及发育具有调控作用。一旦切断毛乳头,毛发将停止生长,然而如果体内形成新的毛乳头,毛囊及毛发将恢复正常生长[9]。毛囊干细胞对于毛囊周期性生长、表皮和皮脂腺的更新换代和皮肤的创伤性修复都至关重要[10]。研究毛发再生,其关键就是改变毛发生长周期,因此如何应用各类药物使毛发尽可能长时间停留在生长期,是广大研究者亟需解决的问题。 近年来,各个领域对于自噬的研究正如火如荼地进行,并且自噬与许多生理性活动息息相关,有许多迹象表明自噬与毛发生长周期之间存在强相关性,其中生物节律和心理应激压力均可作为两者相互联系的中介。 机体内的生物节律活动变化可以一定程度上控制毛囊由生长期转化至退行期,并且AL-NUAIMI等[11]发现如果沉默生物节律相关基因可以延长生长期。最近,有许多研究报道了生物节律与自噬的联系[12]。因此,毛发基质中的自噬活性可能会影响毛囊的生物节律,进而影响毛发周期的发展。 心理压力所诱导的自噬活性的增高可能导致自噬对于毛发生长的诱导能力减弱,从而改变了毛发生长周期。心理压力会通过影响神经激素、神经递质和细胞因子,从而影响毛发周期[13]。慢性束缚应激所诱导的心理压力,可以通过神经肽物质P(neuropeptide substance P,SP)诱导活性氧增加从而改变毛发的生长周期;慢性束缚应激也可以通过SP和活性氧信号通路调节,诱导皮肤中的自噬活性增高,WANG等[14]研究表明慢性束缚应激可能通过激活自噬诱导毛发生长延迟。慢性束缚应激作为一种广泛应用的心理压力应激模型,可延长退行期以抑制毛发生长,自噬活性的改变是二者相关的重要桥梁。 自噬在真核生物的溶酶体降解系统中极为重要,在维持细胞的功能性和完整性方面发挥着重要作用,最终有助于维持细胞内稳态[15]。同时,自噬也是细胞死亡的一种形式[16],是一种程序性细胞死亡,自噬基因水平的增高是细胞死亡的部分原因。自噬可以清除细胞内受损的脂质、蛋白质以及其他物质,也可以在缺氧或营养缺乏等极端条件下保护细胞,影响生物体内多种生理和病理活动,如生物体发育、肿瘤抑制、抗原呈递、生存、死亡及衰老等[17-18]。自噬对生物体内生理及病理活动的调节,主要是研究自噬活性的改变对于各类活动的影响及作用机制。 恰当且适度的自噬可以清除细胞内废弃的蛋白质及细胞器,从而维持细胞内稳态,有利于细胞生存,其与代谢之间的相互影响呈现出了一种复杂的动态反馈回路机制,该机制调控细胞的能量状态,起到保护细胞的作用[19]。自噬还可在胁迫条件下维持细胞存活,营养耗竭所诱导的自噬可提供氨基酸合成所必需的蛋白质,从而延长细胞存活时间[20],除此之外,自噬也可抵消细胞凋亡带来的不利影响[21]。适度自噬对细胞生存是有利的,然而自噬激活过度可能会给细胞带来损害,过早结束细胞存活进程。此外,过度亢进的自噬会导致过度清除细胞内容物,从而抑制细胞功能[22]。活性氧诱导自噬的作用可能取决于自噬的程度,已有研究证明,活性氧轻度诱导的自噬可能具有保护细胞的作用,但是活性氧大量诱导自噬则可能导致过度自噬,以至于消化细胞成分,导致细胞的死亡[23]。毛发生长与生物体的发育和衰老密不可分,因此自噬也可以调节毛发生长,改变其生长周期,如图5所示。"
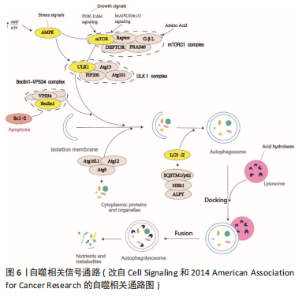
自噬是人体生理性脱发过程中的重要保护机制,可以阻滞毛囊干细胞的衰老和耗竭[24],ASAKAWA等[25]通过过表达小鼠Wnt1基因,使自噬活性受抑而脱发,因此诱导自噬可逆转脱发现象。王雷[26]研究结果表明,毛囊生长周期与自噬活性之间存在高度相关性,表明自噬可能在慢性束缚应激所诱导的抑制毛发生长的表型中起着一定的作用。近期CHAI等[27]在人头皮毛囊器官培养模型中,可观察处于生长期的毛发中有活跃的自噬流(LC3B和p62),且随着毛发由生长期进入退行期,自噬流明显呈现出减弱的趋势[28-29]。因此,维持一定的自噬活性对于人类毛发周期性生长是必不可少的。 现已发现,改变自噬活性可以导致毛发相关疾病,可通过改变自噬活性逆转脱发疾病。许多研究表明,使自噬处于一定水平的活性是维持毛囊正常生长的关键因素,因为过度激活自噬可能会延长毛囊的休止期,延迟其进入生长期。LIU等[30]通过临床试验发现,雄激素性脱发患者秃顶处的微型毛囊处于退行期早期,而且其内源性自噬严重受损,同时伴有细胞凋亡增加,表明可能由于自噬活性减弱且细胞凋亡增加导致雄激素性脱发的发生。FORTES等[31]通过临床研究表明,地中海饮食有助于降低雄激素性脱发的风险,羟基酪醇是其中的主要成分,可激活自噬,从而缓解氧化应激损伤,羟基酪醇通过增强自噬活性、抑制炎症因子的表达和上调毛乳头细胞中毛发生长因子的表达,缓解氧化应激损伤,从而有利于毛发生长[32]。然而,MARI?O等[33]发现,雄激素性脱发患者的雄激素含量高于正常人群,雄激素中的睾酮可以产生二氢睾酮,雄激素性脱发的机制可能与二氢睾酮通过阻断mTORC1和Raptor相互作用从而激活自噬有关,导致自噬的过度激活,这与之前LIU等的观点截然相反,表明过度激活自噬是雄激素性脱发发生的潜在机制。有课题组发现,经芽孢杆菌处理后的菱果所提取出的AC2肽可以通过特异性抑制mTORC1复合物裂解,从而抑制人真皮乳头细胞中二氢睾酮相关的应激[34],维持自噬在相对适度的水平,促进人毛乳头细胞细胞增殖和功能恢复,从而逆转雄激素性脱发带来的脱发现象。丹参酮胶囊与AC2肽相同,可能也是通过抑制自噬活性使毛囊处于适宜生长的自噬水平,促进休止期毛囊进入生长期,延长生长期从而改善雄激素性脱发[35]。自噬活性异常除了可以导致雄激素性脱发,还可以导致其他脱发疾病,如斑秃等。 GUND等[36]发现,自噬蛋白SQSTM1(p62)在斑秃C57BL/6J小鼠的毛囊中积累,表明斑秃小鼠毛囊的自噬活性明显受到抑制,他们还发现 Tat-BECN1可以通过激活自噬活性减弱斑秃的程度,而使用自噬抑制剂氯喹治疗会促进小鼠发生斑秃。使自噬维持适度活性是治疗脱发疾病的关键,然而对于自噬活性的把握却十分困难。 在转染Htr2A/Omi基因的Mnd2小鼠的表皮细胞中也会出现过度自噬,从而导致小鼠出现休止期脱发和体质量减轻的现象[37]。在中医穴位研究中也有所发现,针刺足三里可明显上调心脏、肌肉、肝脏的自噬水平,对老年鼠行该针法,发现能够上调老年小鼠自噬信号通路,促进毛发生长及提高运动能力[38]。郑瑜等[39]研究通过改变自噬活性在气管肺发育不良疾病中的治疗作用,且发现气管肺发育不良模型组的自噬活性显著高于对照组,且动物毛发也呈现出粗糙、蓬乱、光泽差等特点。提示自噬若被过度激活,则不利于细胞生长,抑制毛囊进入生长期,或使毛囊长期处于退行期,不利于其生长与再生。 由上可知,若需要促进毛发生长,适度的自噬是必不可缺的,因此如何使自噬维持在合适的水平是促进正常人群和被脱发疾病所困扰的患者所亟需解决的问题。 2.2 自噬相关基因与信号通路和毛发生长关系 自噬相关基因的表达与否与细胞存活及细胞寿命之间具有相关性。自噬对于细胞存活的重要性,通过缺乏自噬相关基因的生物体或细胞更易死亡也可观察得出,如缺乏自噬蛋白Beclin1的小鼠在早期胚胎发育过程中死亡,而缺乏自噬相关基因Atg5的小鼠在出生一天内就会死亡。小鼠神经细胞缺乏Atg5或Atg7都会导致与年龄相关的神经退化,且寿命缩短。然而自噬也可促进细胞死亡,在Cont3-耗尽的小鼠心脏中,Atg7 mRNA水平升高引起自噬水平增高,导致心肌细胞死亡和心力衰竭,然而敲除Atg7可以提高生存率和心脏功能[40]。在球体突变的卵巢中,增加自噬相关基因Atg12 mRNA表达会增加细胞死亡率,而降低Atg12或其他自噬相关基因的mRNA水平会抑制细胞死亡[41]。过表达Atg5可减少黑色素瘤细胞的增殖,并且诱导衰老,抑制自噬可以延缓癌基因所诱导的衰老的发生[42]。自噬活性的维持,有利于延缓细胞衰老及促进细胞生存和再生。 通过敲除或过表达自噬相关基因可以改变自噬活性,从而影响毛发生长状态,改变毛囊周期。OSANAI等[43]在慢性细胞内酸中毒的小鼠模型上研究延缓衰老的药物时,发现了该模型下的小鼠都表现出寿命缩短和早衰的相关表型,其中脱发就位列其一,原因在于自噬相关基因Atg7的乙酰化导致的自噬活性降低。Atg7对自噬体的形成十分重要,缺乏Atg7的黑色素细胞会呈现出过早衰老和抗氧化系统失调等现象[44],YOSHIHARA等[45]将该基因缺陷的小鼠植入皮肤移植物后,发现了促进毛乳头细胞生长的异常现象,但是其生长速度比对照组慢。近期,有研究发现颗粒物在体内和体外均可以诱导毛囊自噬通量增加,人类毛囊器官培养中使用siRNA敲除Atg5,自噬受抑制可能会增加颗粒物所引起的细胞毒性和提前结束退行期的可能性,表明自噬可能是颗粒物引起脱发的体内潜在防御机制[46]。在斑秃疾病中,也可发现自噬与该疾病的关联,对96例斑秃患者和正常人群的头皮活检进行基因表达分析,观察到自噬相关基因Atg4B被拷贝数变异(CNVs)所影响,表明自噬失调可以引起斑秃[47]。PETUKHOVA 等[48]通过全基因组关联分析(GWAS分析)已经将两条自噬相关通路(PARK2和PFKFB3)与斑秃易感性联系了起来。斑秃患者毛囊的毛发基质相比于正常人群,其自噬标志物Atg5和LC3B的蛋白质表达水平明显降低,说明斑秃患者的毛囊基质中的自噬活性受抑制[49]。转化生长因子TGFβ-1和TGFβ-2不仅是毛囊免疫能力的守护者[50],还可以参与自噬的调控[51]。γ-干扰素可以驱动TGFβ-2,促使人类头发过早进入退行期和营养不良,γ-干扰素是参与斑秃发病的重要细胞因子[52],可以显著减少毛囊滤泡内的自噬通量[49],说明γ-干扰素可能是通过降低自噬活性从而引发斑秃的潜在机制。 图6为自噬相关的信号通路。近期研究发现,自噬可能通过磷脂酰肌醇3-激酶(phosphatidylinositol 3 kinase,PI3K)-Akt-mTOR信号通路作用于毛发生长过程,SCD1作为其下游效应分子,罗怡[53]研究表明SCD1可能可以通过该信号通路促进小鼠毛囊细胞的细胞增殖和抑制细胞自噬对毛发生长的保护作用。有研究表明,雄激素性脱发中的PTEN、PI3K/Akt、MAPK、Wnt、JAK/STAT等信号通路异常,并且这些通路与自噬的关系十分密切[28,54]。肉豆蔻油酸(MA)通过自噬的途径激活真皮乳头间叶细胞(DP细胞)中的Wnt/β-catenin和ERK信号通路,从而促进生长期的信号传导[55],因此Wnt/β-catenin和ERK信号通路可能位于自噬的信号通路下游。"
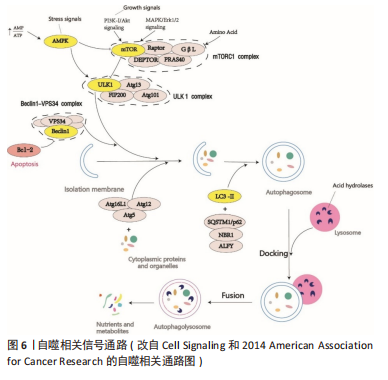
除此之外,TGF-β家族的成员之一——骨形态发生蛋白(BMP2),在骨形成、再生和胚胎形成中发挥重要作用[56],通过TGF-β信号通路调节可以在神经干细胞中激活毛囊生态位,促进毛发生长[57]。PTEN可以从细胞质转移至由氧化应激所诱导的细胞核中,进而导致自噬[58]。CAI等[59]在小鼠创伤模型中,发现骨形态发生蛋白2可以上调毛囊干细胞中的PTEN和自噬相关蛋白表达,说明骨形态发生蛋白2可以增强PTEN的表达并且诱导自噬,从而促进毛囊干细胞的分化、伤口收缩和表皮再生。 除了以上提及的信号通路可以调节自噬活性进而改变毛囊生长状态,成纤维细胞生长因子(FGF)的信号传导可以正向调节软骨细胞的自噬,甚至可以参与毛发生长周期的调节[60]。毛囊生长期中主要表达角质形成细胞生长因子(FGF7),可以保护人类毛囊免受紫外线照射、化疗或药物诱导的细胞死亡[61],同时,成纤维细胞生长因子5的信号传导可以调控毛囊退行期[60]。因此,成纤维细胞生长因子信号传导程度是可以反映出毛囊器官培养中自噬活性的,可以通过改变成纤维细胞生长因子的信号传导程度,从而改变自噬活性,使其维持在适于毛发生长的范围内。 自噬相关基因及信号通路的转导与毛发生长密不可分,应用各类抑制剂与激活剂通过相关信号通路改变自噬活性,从而使其维持在适宜的程度,进而使毛囊加快进入或延长生长期,最终利于毛发生存,逆转脱发患者的脱发现象。 2.3 自噬激活剂与抑制剂和毛发生长的关系 自噬的激活剂与抑制剂,如表1所示,通过作用于不同通路或因子,激活或抑制自噬通量完成对于机体各个系统状态的调控,其中就包括对于毛发生长的调控,可以使其维持在适宜生长的状态。"
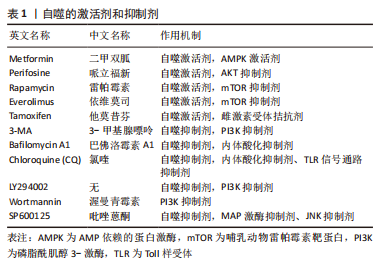
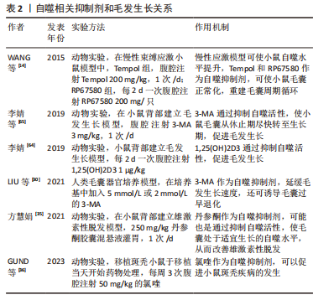
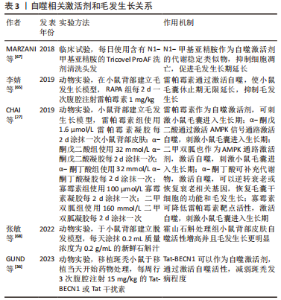
2.3.1 自噬抑制剂与毛发生长的关系 TOSTI等[62]最初发现抗疟药氯喹是自噬的抑制剂,其不利于毛发生长,出现休止期脱发的现象,但是氯喹可能通过不依赖自噬的方式影响毛发周期[63]。此外,有课题组发现,给予1,25(OH)2D3后,小鼠的毛囊上皮细胞中的增殖标记物Ki67明显增加,并且毛囊干细胞标志物CD34和CD49f表达也明显增加[64]。给予自噬抑制剂3-MA的效果与1,25(OH)2D3的效果相同;李倩等[65]也发现了使用自噬抑制剂3-MA抑制自噬,可以促进小鼠毛囊从休止期转化为生长期,这表明自噬在毛发生长周期中确实发挥着重要作用。LIU等[30]在毛囊器官培养中加入3-MA,发现可以在减缓毛发生长速度的同时,诱导细胞凋亡和毛囊过早退化。而WANG等[14]使用自噬抑制剂Tempol和RP67580,发现可以抑制毛囊细胞中自噬相关蛋白的表达,使慢性应激模型的小鼠毛囊正常化,从而对于毛囊循环周期进行重建。相比于自噬抑制剂,自噬激活剂的应用则更为广泛。表2为与毛发生长相关的自噬抑制剂。"
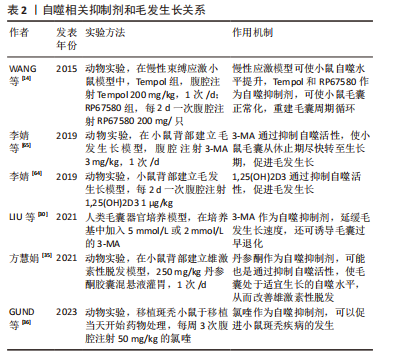
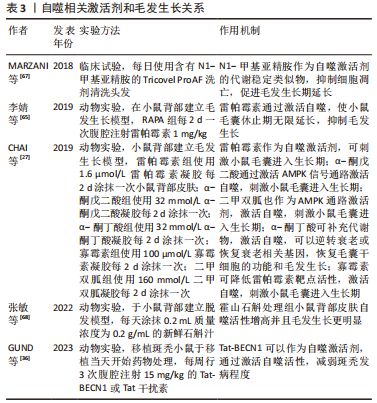
2.3.2 自噬激活剂与毛发生长的关系 最初,VISHNIAKOVA等[24]发现,促毛发生长剂(PSHG)的促进毛发生长作用很有可能是通过促进小鼠皮肤的自噬活性,从而清除了衰老和损伤的毛囊细胞。现在市面上常使用的一款抗脱发产品的主要成分包括生物素和N1-甲基亚精胺,且后者是公认的卵泡内自噬激活剂的代谢稳定类似物[66],可以通过抑制毛囊细胞凋亡来延长生长期[28]。MARZANI等[67]在临床试验中,同样证实了N1-甲基亚精胺的安全性和防脱发的功效,并且PARODI等[28]在毛囊器官培养模型中,发现这些抗脱发产品可显著增强毛囊内的自噬活性,导致生长期的延长。近期,张敏等[68]在研究霍山石斛促进毛发生长的机制时,发现用霍山石斛处理小鼠背部皮肤,可使皮肤组织的自噬活性增高和细胞生长分化因子相关基因表达下降,并且与对照组相比,处理后的小鼠背部皮肤的毛发生长更明显。相比于自噬抑制剂大部分作用于病理性脱发疾病,自噬激活剂对于生理性脱发有明显的促进毛发再生的作用。 自噬激活剂在毛发生长中的相关研究是从近十年来才逐步发展,而且如表3所示,其对于毛囊生长作用的影响与给药方式和剂量均有关。CHAI等[27]在C57BL/6J小鼠背部皮肤模型中,检测一些小分子是否可以参与代谢和诱导自噬,从而进行毛发生长的调节,有趣的是,其中对于雷帕霉素(RAPA)的作用与前文叙述不同。在小鼠模型中,将雷帕霉素制作为凝胶涂抹在小鼠背部皮肤,可以刺激毛囊尽快进入生长期[27]。除此之外,他们还使用α-酮戊二酸(α-KG)、α-酮丁酸(α-KB)、寡霉素和二甲双胍在小鼠背部进行局部治疗。α-酮戊二酸作为AMPK信号通路的下游复合物[69],可以在葡萄糖饥饿和许多其他应激条件下被激活[70],进而激活自噬[71]。二甲双胍是AMPK通路的激活剂[72],广泛用于治疗糖尿病,是第一个获准用于研究人类抗衰老的药物[73]。已有研究表明,使用二甲双胍治疗多囊卵巢综合征的患者,可以减轻其脱发症状[74-75] 。α-酮丁酸可以延长老年小鼠的寿命,并且减轻许多与衰老相关的症状。CHAI等[27]使用这些药物对小鼠进行局部治疗后,均可刺激毛囊从休止期转化为生长期,从而促进毛发生长;并且α-酮戊二酸促进毛发生长的作用,可被自噬阻断剂所阻断。"
| [1] 吴大兴,杨松标,钮正祥,等.斑秃患者与雄激素性脱发患者心理状况比较[J].预防医学,2017,29(5):511-513+517. [2] 邱学军,龙俊茹,韩亮,等.斑秃非手术治疗法的研究现状与展望[J].激光生物学报,2022,31(3):208-214. [3] CLAUDE-TAUPIN A, DUPONT N, CODOGNO P. Autophagy and the primary cilium in cell metabolism: What’s upstream? Front Cell Dev Biol. 2022;10:1046248. [4] FERNáNDEZ ÁF, SEBTI S, WEI Y, et al. Disruption of the beclin 1-BCL2 autophagy regulatory complex promotes longevity in mice. Nature. 2018;558(7708):136-140. [5] SAKAMAKI JI, RYAN KM. Autophagy Determines the Path on the TRAIL to Death. Dev Cell. 2016;37(4):291-293. [6] PATTINGRE S, TASSA A, QU X, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122(6):927-939. [7] 郭雪峰,包鹏甲,常永芳,等.哺乳动物毛囊发育及调控研究进展[J].中国畜牧兽医,2019,46(2):387-394. [8] ZHAO R, YIHAN W, ZHAO Y, et al. Hair follicle regional specificity in different parts of bay Mongolian horse by histology and transcriptional profiling. BMC Genomics. 2020;21(1):651. [9] 王缓,王兴群,邹希,等.宣肺视角下脱发炎性因子的表达改变[J].中国中医药现代远程教育,2022,20(12):202-205. [10] 贾琪,刘艳光,罗新惠,等.毛囊干细胞主要信号分子调控研究进展[J].延边大学农学学报,2021,43(3):101-108. [11] AL-NUAIMI Y, HARDMAN JA, BÍRÓ T, et al. A meeting of two chronobiological systems: circadian proteins Period1 and BMAL1 modulate the human hair cycle clock. J Invest Dermatol. 2014;134(3):610-619. [12] DA ROCHA AL, PINTO AP, BEDO BLS, et al. Exercise alters the circadian rhythm of REV-ERB-α and downregulates autophagy-related genes in peripheral and central tissues. Sci Rep. 2022;12(1):20006. [13] ZHANG J, CHEN R, WEN L, et al. Recent Progress in the Understanding of the Effect of Sympathetic Nerves on Hair Follicle Growth. Front Cell Dev Biol. 2021;9: 736738. [14] WANG L, GUO LL, WANG LH, et al. Oxidative stress and substance P mediate psychological stress-induced autophagy and delay of hair growth in mice. Arch Dermatol Res. 2015;307(2):171-181. [15] KAO WC, CHEN JC, LIU PC, et al. The Role of Autophagy in Osteoarthritic Cartilage. Biomolecules. 2022;12(10):1357. [16] YOSHIMOTO T, MORINE Y, TAKASU C, et al. Blue light-emitting diodes induce autophagy in colon cancer cells by Opsin 3. Ann Gastroenterol Surg. 2018;2(2): 154-161. [17] DIKIC I, ELAZAR Z. Mechanism and medical implications of mammalian autophagy. Nat Rev Mol Cell Biol. 2018;19(6):349-364. [18] LEVINE B, KROEMER G. Biological Functions of Autophagy Genes: A Disease Perspective. Cell. 2019;176(1-2):11-42. [19] YU JE, KIM Y, HONG DE, et al. Bee Venom Triggers Autophagy-Induced Apoptosis in Human Lung Cancer Cells via the mTOR Signaling Pathway. J Oncol. 2022;2022: 8916464. [20] D’AMICO R, IMPELLIZZERI D, CORDARO M, et al. Complex Interplay between Autophagy and Oxidative Stress in the Development of Endometriosis. Antioxidants (Basel). 2022;11(12):2484. [21] GEORGIOU M, YANG C, ATKINSON R, et al. Activation of autophagy reverses progressive and deleterious protein aggregation in PRPF31 patient-induced pluripotent stem cell-derived retinal pigment epithelium cells. Clin Transl Med. 2022;12(3):e759. [22] GUO J, ZHU K, LI Z, et al. Adiponectin Protects Hypoxia/Reoxygenation-Induced Cardiomyocyte Injury by Suppressing Autophagy. J Immunol Res. 2022;2022:8433464. [23] ABID R, GHAZANFAR S, FARID A, et al. Pharmacological Properties of 4’, 5, 7-Trihydroxyflavone (Apigenin) and Its Impact on Cell Signaling Pathways. Molecules. 2022;27(13):4304. [24] VISHNIAKOVA KS, POPOV KV, VOROTELIAK EA, et al. Possible role of autophagy activation in the stimulation of regeneration. Mol Biol (Mosk). 2013;47(5):796-805. [25] ASAKAWA K, TOYOSHIMA KE, TSUJI T. Functional Hair Follicle Regeneration by the Rearrangement of Stem Cells. Methods Mol Biol. 2017;1597:117-134. [26] 王雷.氧化应激调节自噬参与C57BL/6小鼠慢性束缚应激诱导的毛囊生长期延迟[D].苏州:苏州大学,2013. [27] CHAI M, JIANG M, VERGNES L, et al. Stimulation of Hair Growth by Small Molecules that Activate Autophagy. Cell Reports. 2019;27(12):3413-3421. [28] PARODI C, HARDMAN JA, ALLAVENA G, et al. Autophagy is essential for maintaining the growth of a human (mini-)organ: Evidence from scalp hair follicle organ culture. PLoS Biology. 2018;16(3):e2002864. [29] KLIONSKY DJ, ABDELMOHSEN K, ABE A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy. 2016;12(1):1-222. [30] LIU W, LI K, WANG G, et al. Impairment of autophagy may be associated with follicular miniaturization in androgenetic alopecia by inducing premature catagen. J Dermatol. 2021;48(3):289-300. [31] FORTES C, MASTROENI S, MANNOORANPARAMPIL T, et al. Mediterranean diet: fresh herbs and fresh vegetables decrease the risk of Androgenetic Alopecia in males. Arch Dermatol Res. 2018;310(1):71-76. [32] 陈倩. 羟基酪醇调控自噬缓解毛乳头细胞氧化应激损伤机制研究[D].广州:南方医科大学,2019. [33] MARIÑO G, NISO-SANTANO M, BAEHRECKE EH, et al. Self-consumption: the interplay of autophagy and apoptosis. Nat Re Mol Cell Biol. 2014;15(2):81-94. [34] NAM GH, JO KJ, PARK YS, et al. The peptide AC 2 isolated from Bacillus-treated Trapa japonica fruit extract rescues DHT (dihydrotestosterone)-treated human dermal papilla cells and mediates mTORC1 signaling for autophagy and apoptosis suppression. Sci Rep. 2019;9(1):16903. [35] 方慧娟.丹参酮胶囊治疗雄激素性秃发模型小鼠的机制研究[D]. 北京:北京中医药大学,2021. [36] GUND R, CHRISTIANO AM. Impaired autophagy promotes hair loss in the C3H/HeJ mouse model of alopecia areata. Autophagy. 2023;19(1):296-305. [37] KANG S, LOUBOUTIN JP, DATTA P, et al. Loss of HtrA2/Omi activity in non-neuronal tissues of adult mice causes premature aging. Cell Death Differ. 2013;20(2):259-269. [38] 林承列.针刺足三里穴位调节细胞自噬的研究[D].成都:成都中医药大学, 2017. [39] 郑瑜,厉兰,胡绘平,等.自噬体及凋亡小体对新生小鼠支气管肺发育不良调控作用[J].河北医科大学学报,2020,41(11):1296-1300. [40] YAMAGUCHI T, SUZUKI T, SATO T, et al. The CCR4-NOT deadenylase complex controls Atg7-dependent cell death and heart function. Sci Signal. 2018;11(516):eaan3638. [41] FANG J, LERIT DA. Orb-dependent polyadenylation contributes to PLP expression and centrosome scaffold assembly. Development. 2022;149(13):dev200426. [42] KLAPAN K, SIMON D, KARAULOV A, et al. Autophagy and Skin Diseases. Front Pharmacol. 2022;13:844756. [43] OSANAI T, TANAKA M, IZUMIYAMA K, et al. Intracellular protons accelerate aging and switch on aging hallmarks in mice. J Cell Biochem. 2018;119(12):9825-9837. [44] ZHANG CF, GRUBER F, NI C, et al. Suppression of autophagy dysregulates the antioxidant response and causes premature senescence of melanocytes. J Invest Dermatol. 2015;135(5):1348-1357. [45] YOSHIHARA N, UENO T, TAKAGI A, et al. The significant role of autophagy in the granular layer in normal skin differentiation and hair growth. Arch Dermatol Res. 2015;307(2):159-169. [46] YU DA, JANG S, OHN J, et al. Protective effect of autophagy in particulate matter-induced hair loss. J Dermatol Sci. 2022;107(3):173-176. [47] PETUKHOVA L, PATEL AV, RIGO RK, et al. Integrative analysis of rare copy number variants and gene expression data in alopecia areata implicates an aetiological role for autophagy. Exp Dermatol. 2020;29(3):243-253. [48] PETUKHOVA L, DUVIC M, HORDINSKY M, et al. Genome-wide association study in alopecia areata implicates both innate and adaptive immunity. Nature. 2010; 466(7302):113-117. [49] HARDMAN JA, NICU C, TAI C, et al. Does dysfunctional autophagy contribute to immune privilege collapse and alopecia areata pathogenesis? J Dermatol Sci. 2020;100(1):75-78. [50] XIE B, SUN J, SONG X. Hair Follicle Melanocytes Initiate Autoimmunity in Alopecia Areata: a Trigger Point. Clin Rev Allergy Immunol. 2022;63(3):417-430. [51] ZHANG Y, ALEXANDER PB, WANG XF. TGF-β Family Signaling in the Control of Cell Proliferation and Survival. Cold Spring Harb Perspect Biol. 2017;9(4):a022145. [52] BERTOLINI M, MCELWEE K, GILHAR A, et al. Hair follicle immune privilege and its collapse in alopecia areata. Exp Dermatol. 2020;29(8):703-725. [53] 罗怡.SCD1通过抑制自噬对毛囊生长的调控作用及机制研究[D].重庆:重庆医科大学,2022. [54] LOLLI F, PALLOTTI F, ROSSI A, et al. Androgenetic alopecia: a review. Endocrine. 2017;57(1):9-17. [55] CHOI YK, KANG JI, HYUN JW, et al. Myristoleic Acid Promotes Anagen Signaling by Autophagy through Activating Wnt/β-Catenin and ERK Pathways in Dermal Papilla Cells. Biomol Ther (Seoul). 2021;29(2):211-219. [56] ROBERTO VP, GAVAIA P, NUNES MJ, et al. Evidences for a New Role of miR-214 in Chondrogenesis. Sci Rep. 2018;8(1):3704. [57] HWANG I, CHOI KA, PARK HS, et al. Neural Stem Cells Restore Hair Growth Through Activation of the Hair Follicle Niche. Cell Transplant. 2016;25(8):1439-1451. [58] GONZÁLEZ-GARCíA A, GARRIDO A, CARRERA AC. Targeting PTEN Regulation by Post Translational Modifications. Cancers (Basel). 2022;14(22):5613. [59] CAI B, ZHENG Y, YAN J, et al. BMP2-mediated PTEN enhancement promotes differentiation of hair follicle stem cells by inducing autophagy. Exp Cell Res. 2019;385(2):111647. [60] SCHNEIDER MR, SCHMIDT-ULLRICH R, PAUS R. The hair follicle as a dynamic miniorgan. Curr Biol. 2009;19(3):R132-R142. [61] BRAUN S, KRAMPERT M, BODÓ E, et al. Keratinocyte growth factor protects epidermis and hair follicles from cell death induced by UV irradiation, chemotherapeutic or cytotoxic agents. J Cell Sci. 2006;119(Pt 23):4841-4849. [62] TOSTI A, PAZZAGLIA M. Drug reactions affecting hair: diagnosis. Dermatol Clin. 2007;25(2):223-231. [63] LIANG DH, CHOI DS, ENSOR JE, et al. The autophagy inhibitor chloroquine targets cancer stem cells in triple negative breast cancer by inducing mitochondrial damage and impairing DNA break repair. Cancer Lett. 2016;376(2):249-258. [64] 李婧.1,25(OH)_2D_3对C57BL/6小鼠毛发再生的作用研究[D].重庆:重庆医科大学,2019. [65] 李婧,罗福玲,吴盛旺,等.3-甲基腺嘌呤通过抑制自噬促进小鼠毛囊再生的作用研究[J].中国药科大学学报,2019,50(4):468-474. [66] CERVELLI M, AVERNA M, VERGANI L, et al. The Involvement of Polyamines Catabolism in the Crosstalk between Neurons and Astrocytes in Neurodegeneration. Biomedicines. 2022;10(7):1756. [67] MARZANI B, PINTO D, SORBELLINI E, et al. New multi-targeting strategy in hair growth promotion: in vitro and in vivo studies. G Ital Dermatol Venereol. 2018;153(3):338-343. [68] 张敏,黄蓉,段亚君,等.霍山石斛通过激活自噬和抑制凋亡促进脱发模型小鼠生发作用[J].合肥工业大学学报(自然科学版),2022,45(6):844-848. [69] CHEN T, MA J, XU C, et al. Increased ATPase activity promotes heat-resistance, high-yield, and high-quality traits in rice by improving energy status. Front Plant Sci. 2022;13:1035027. [70] MIYAZAWA H, SNAEBJORNSSON MT, PRIOR N, et al. Glycolytic flux-signaling controls mouse embryo mesoderm development. Elife. 2022;11:e83299. [71] HU T, CHEN X, LU S, et al. Biological Role and Mechanism of Lipid Metabolism Reprogramming Related Gene ECHS1 in Cancer. Technol Cancer Res Treat. 2022;21:15330338221140655. [72] XU Z, YE H, XIAO W, et al. Metformin Attenuates Inflammation and Fibrosis in Thyroid-Associated Ophthalmopathy. Int J Mol Sci. 2022;23(24):15508. [73] VINCIGUERRA M, OLIER I, ORTEGA-MARTORELL S, et al. New use for an old drug: Metformin and atrial fibrillation. Cell Rep Med. 2022;3(12):100875. [74] OU HT, CHEN PC, WU MH, et al. Metformin improved health-related quality of life in ethnic Chinese women with polycystic ovary syndrome. Health Qual Life Outcomes. 2016;14(1):119. [75] SHAHEBRAHIMI K, JALILIAN N, BAZGIR N, et al. Comparison clinical and metabolic effects of metformin and pioglitazone in polycystic ovary syndrome. Indian J Endocrinol Metab. 2016;20(6):805-809. [76] LIN RL, GARIBYAN L, KIMBALL AB, et al. Systemic causes of hair loss. Ann Med. 2016;48(6):393-402. [77] TRIPATHI M, SINGH BK, LIEHN EA, et al. Caffeine prevents restenosis and inhibits vascular smooth muscle cell proliferation through the induction of autophagy. Autophagy. 2022;18(9):2150-2160. [78] FISCHER TW, HERCZEG-LISZTES E, FUNK W, et al. Differential effects of caffeine on hair shaft elongation, matrix and outer root sheath keratinocyte proliferation, and transforming growth factor-β2/insulin-like growth factor-1-mediated regulation of the hair cycle in male and female human hair follicles in vitro. Br J Dermatol. 2014;171(5):1031-1043. |
| [1] | Liu Qiwei, Zhang Junhui, Yang Yuan, Wang Jinjuan. Role and mechanism of umbilical cord mesenchymal stem cells on polycystic ovary syndrome [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1015-1020. |
| [2] | Pan Xiaolong, Fan Feiyan, Ying Chunmiao, Liu Feixiang, Zhang Yunke. Effect and mechanism of traditional Chinese medicine on inhibiting the aging of mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1091-1098. |
| [3] | Xue Jingwen, Wang Fangfang, Zhang Xin, Pang Ruifeng, Wang Xiaoye, Ma Xiaoru. Effect of ganoderma spore on mitochondrial autophagy and apoptosis in testicular tissue of diabetic rats [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 562-568. |
| [4] | Yan Binghan, Li Zhichao, Su Hui, Xue Haipeng, Xu Zhanwang, Tan Guoqing. Mechanisms of traditional Chinese medicine monomers in the treatment of osteoarthritis by targeting autophagy [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 627-632. |
| [5] | Wang Jingfeng, Wen Dengtai, Wang Shijie, Gao Yinghui. Atg-mediated autophagy, exercise and skeletal muscle aging [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(2): 295-301. |
| [6] | Ma Suilu, He Zhijun, Liu Tao, Li Yan, He Yuanxu, He Bo, Wang Weiwei, Wei Xiaotao. Traditional Chinese medicine monomer in the prevention and treatment of flap necrosis by regulating “autophagy” [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(1): 153-158. |
| [7] | Deng Rui, Huang Keming, Luo Jian, Chen Gong, Feng Jian, Huang Weiyi, Wei Gang. Effect of heme oxygenase-1-mediated atorvastatin on macrophage polarization and cholesterol accumulation [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(1): 62-67. |
| [8] | Nie Chenchen, Su Kaiqi, Gao Jing, Fan Yongfu, Ruan Xiaodi, Yuan Jie, Duan Zhaoyuan, Feng Xiaodong. The regulatory role of circular RNAs in cerebral ischemia-reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1286-1291. |
| [9] | Li Long, Li Guangdi, Shi Hao, Deng Keqi. Circular RNA as a competing endogenous RNA is involved in the regulation of osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(5): 751-757. |
| [10] | Li Zhichao, Tan Guoqing, Su Hui, Xu Zhanwang, Xue Haipeng. Regulatory role of non-coding RNAs as potential therapeutic targets in spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(5): 758-764. |
| [11] | Wu Yujie, Wan Xiaofang, Wei Mianxing, Peng Shiyuan, Xu Xiaomei. Correlation between autophagy and the Hippo-YAP protein pathway in periodental ligament cells on the pressure side of a mouse model of orthodontic tooth movement [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(5): 683-689. |
| [12] | Jin Hongguang, Shi Zhuang, Wang Huaxin, Ni Ma, Fu Yong. Mechanism of Mongolian Medicine Erden-uril on avascular necrosis of the femoral head in rats [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(35): 5670-5675. |
| [13] | Gu Yingchu, Gu Ye, Wu Zerui, Fang Tao, Wang Qiufei, Chen Bingqian, Peng Yuqin, Geng Dechun, Xu Yaozeng. Signaling pathway of osteoblast autophagy in periprosthetic osteolysis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(34): 5561-5569. |
| [14] | Tang Li, Pan Yao, Zhu Guochen. Epidermal neural crest stem cells of hair follicles regulate the local expression of inflammatory factors after facial nerve injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(33): 5249-5255. |
| [15] | Zhang Qi, Li Guilin, He Yanjiao. Ultrastructural features and clinical significance of autophagosome in benign meningiomas [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(33): 5304-5308. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||

