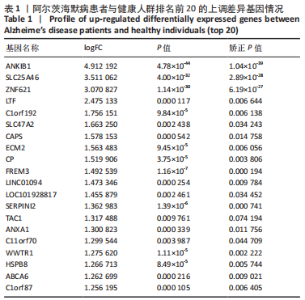
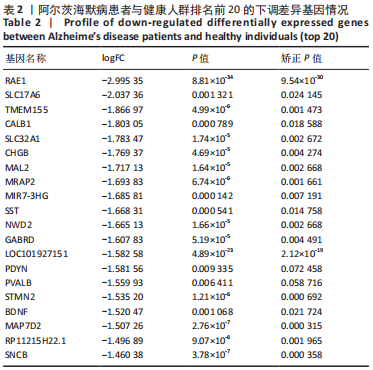
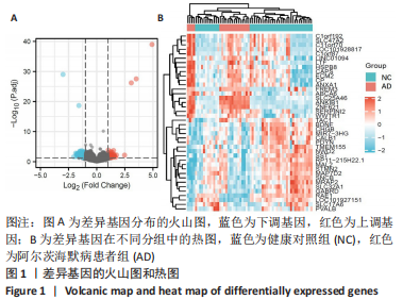
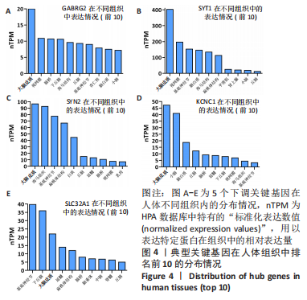
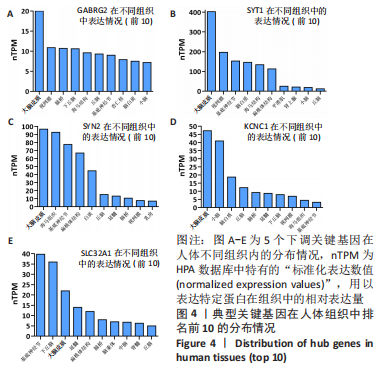
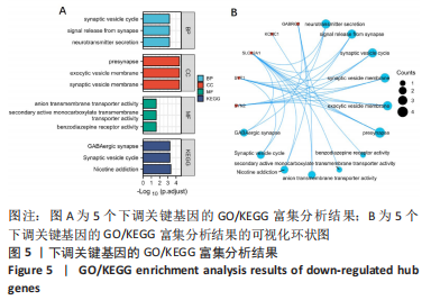
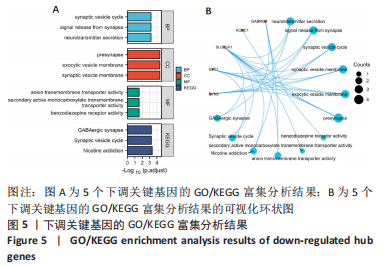
[1] 2022 Alzheimer’s disease facts and figures. Alzheimers Dement. 2022;18(4):700-789.
[2] KOCH I, ANDRADE-NAVARRO M, SCHULZ MH, et al. Bioinformatics in theory and application - highlights of the 36th German Conference on Bioinformatics. Biol Chem. 2021;402(8):869-870.
[3] SCHELTENS P, DE STROOPER B, KIVIPELTO M, et al. Alzheimer’s disease. Lancet. 2021;397(10284):1577-1590.
[4] ZHOU J, LIANG W, WANG J, et al. An epileptic encephalopathy associated GABRG2 missense mutation leads to pre- and postsynaptic defects in zebrafish. Hum Mol Genet. 2022;31(19):3216-3230.
[5] CARPENTER JC, MÄNNIKKÖ R, HEFFNER C, et al. Progressive myoclonus epilepsy KCNC1 variant causes a developmental dendritopathy. Epilepsia. 2021;62(5):1256-1267.
[6] GUERRINI R, MARINI C. SLC32A1: One More Gene Contributing to the Solution of the Genetic Generalized Epilepsies Mystery. Neurology. 2021;96(18):831-832.
[7] HERON SE, REGAN BM, HARRIS RV, et al. Association of SLC32A1 Missense Variants With Genetic Epilepsy With Febrile Seizures Plus. Neurology. 2021; 96(18):e2251-e2260.
[8] WANG S, ZHANG X, ZHOU L, et al. Analysis of GABRG2 C588T polymorphism in genetic epilepsy and evaluation of GABRG2 in drug treatment. Clin Transl Sci. 2021;14(5):1725-1733.
[9] GHIT A, ASSAL D, AL-SHAMI AS, et al. GABAA receptors: structure, function, pharmacology, and related disorders. J Genet Eng Biotechnol. 2021;19(1):123.
[10] 蒋永莉,江文. GABRG2基因突变与癫痫相关性研究进展[J].中华神经科杂志,2020,53(10):824-829.
[11] ZHANG Y, ALI SR, NABBOUT R, et al. A KCNC1 mutation in epilepsy of infancy with focal migrating seizures produces functional channels that fail to be regulated by PKC phosphorylation. J Neurophysiol. 2021;126(2):532-539.
[12] COETZEE WA, AMARILLO Y, CHIU J, et al. Molecular diversity of K+ channels. Ann N Y Acad Sci. 1999;868:233-285.
[13] RANJAN R, LOGETTE E, MARANI M, et al. A Kinetic Map of the Homomeric Voltage-Gated Potassium Channel (Kv) Family. Front Cell Neurosci. 2019;13:358.
[14] GUTMAN GA, CHANDY KG, GRISSMER S, et al. International Union of Pharmacology. LIII. Nomenclature and molecular relationships of voltage-gated potassium channels. Pharmacol Rev. 2005;57(4):473-508.
[15] ALLEN NM, WECKHUYSEN S, GORMAN K, et al. Genetic potassium channel-associated epilepsies: Clinical review of the Kv family. Eur J Paediatr Neurol. 2020;24:105-116..
[16] RUDY B, MCBAIN CJ. Kv3 channels: voltage-gated K+ channels designed for high-frequency repetitive firing. Trends Neurosci. 2001;24(9):517-526.
[17] ERISIR A, LAU D, RUDY B, et al. Function of specific K(+) channels in sustained high-frequency firing of fast-spiking neocortical interneurons. J Neurophysiol. 1999;82(5):2476-2489.
[18] GASNIER B. The SLC32 transporter, a key protein for the synaptic release of inhibitory amino acids. Pflugers Arch. 2004;447(5):756-759.
[19] 李连娥. GABA系统调节对阿尔兹海默病及正常衰老的影响[D].昆明:昆明理工大学,2017.
[20] BRAGINA L, BONIFACINO T, BASSI S, et al. Differential expression of metabotropic glutamate and GABA receptors at neocortical glutamatergic and GABAergic axon terminals. Front Cell Neurosci. 2015;9:345.
[21] NAVA-MESA MO, JIMÉNEZ-DÍAZ L, YAJEYA J, et al. GABAergic neurotransmission and new strategies of neuromodulation to compensate synaptic dysfunction in early stages of Alzheimer’s disease. Front Cell Neurosci. 2014;8:167.
[22] JO S, YARISHKIN O, HWANG YJ, et al. GABA from reactive astrocytes impairs memory in mouse models of Alzheimer’s disease. Nat Med. 2014;20(8):886-896.
[23] TAMBINI MD, YAO W, D’ADAMIO L. Facilitation of glutamate, but not GABA, release in Familial Alzheimer’s APP mutant Knock-in rats with increased β-cleavage of APP. Aging Cell. 2019;18(6):e13033.
[24] LANGLOIS CM, BRADBURY A, WOOD EM, et al. Alzheimer’s Prevention Initiative Generation Program: Development of an APOE genetic counseling and disclosure process in the context of clinical trials. Alzheimers Dement (N Y). 2019;5:705-716.
|