Chinese Journal of Tissue Engineering Research ›› 2023, Vol. 27 ›› Issue (14): 2200-2206.doi: 10.12307/2023.149
Previous Articles Next Articles
Regulatory mechanism of myoblast proliferation and differentiation
Cheng Chunfang1, Wan Juan1, Ding Kaizhi2, Song Jiahao2, Tang Shan1, Gong Yanchun2, Yao Lihua2
- 1Institute of Physical Education, 2School of Life Science; Jiangxi Science & Technology Normal University, Nanchang 330013, Jiangxi Province, China
-
Received:2022-04-14Accepted:2022-05-28Online:2023-05-18Published:2022-09-30 -
Contact:Yao Lihua, MD, Professor, School of Life Science; Jiangxi Science & Technology Normal University, Nanchang 330013, Jiangxi Province, China -
About author:Cheng Chunfang, Master candidate, Institute of Physical Education, Jiangxi Science & Technology Normal University, Nanchang 330013, Jiangxi Province, China -
Supported by:the National Natural Science Foundation of China, Nos. 31960193 and 31660275 (to YLH); Technology Innovation High-end Talents Project of Jiangxi Provincial “Double Thousand Plan”, No. jxsq2019201011 (to YLH)
CLC Number:
Cite this article
Cheng Chunfang, Wan Juan, Ding Kaizhi, Song Jiahao, Tang Shan, Gong Yanchun, Yao Lihua. Regulatory mechanism of myoblast proliferation and differentiation[J]. Chinese Journal of Tissue Engineering Research, 2023, 27(14): 2200-2206.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
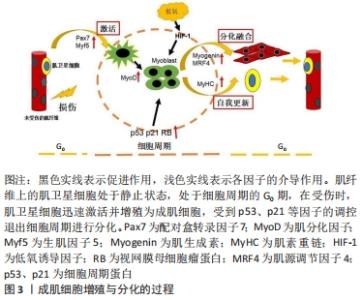
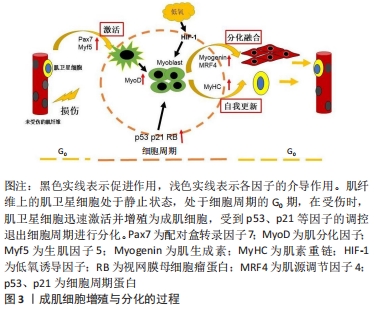
2.1 成肌细胞增殖与分化的过程 成肌细胞增殖与分化是损伤或疾病后骨骼肌发育和再生的基础。在正常的生理状态下,肌卫星细胞处于静息状态,然而伴随着各种生理刺激及病理损伤,肌卫星细胞被激活迅速转化为具有增殖分化能力的成肌细胞,在肌分化因子(myogenic differentiation,MyoD)及生肌因子5 (myogenic factor 5,Myf5)调控下,单核的成肌细胞在肌肉组织中增殖形成一定数量的肌源性细胞群,开始启动分化,成肌细胞受到p53、p21等因子的调控,以不可逆的形式退出细胞周期,经增殖分化及融合形成多核肌管,进而伴随着肌生成素(myogenin,MyoG)、肌源性调节因子4(myogenin Regulatory factor 4,MRF4)、肌素重链(myosin heavy chain,MyHC)等因子的表达,最终形成新的肌纤维,实现修复受损的肌纤维[7-9]。在成肌细胞分化过程中,往往伴随着一系列参与调控细胞周期及其相关信号通路的调控因子表达水平的改变,因此,开展成肌细胞增殖与分化的基因调控研究对于进一步深入解析骨骼肌的损伤修复具有重要的潜在应用价值[10],见图3。"
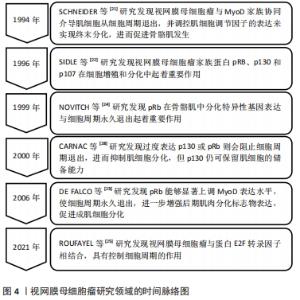
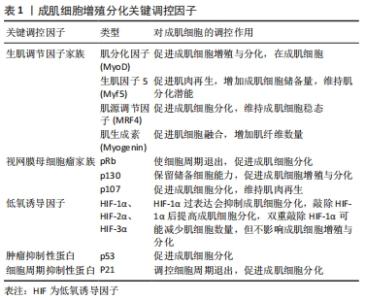
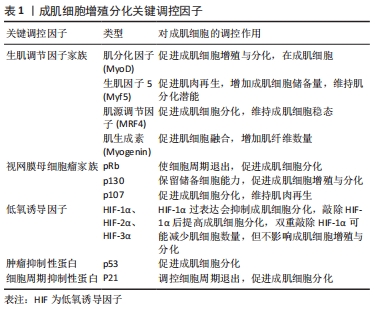
2.2.1 生肌调节因子家族 生肌调节因子(myogenin regulatory factor family,MRFs)家族是调控骨骼肌发生的重要基因,由多种转录因子组成,其中主要包括MyoD、Myf5、肌生成素(Myogenin)、肌源性调节因子4(MRF4),共同参与并调控成肌细胞增殖与分化[11-12]。 MyoD和Myf5是骨骼肌损伤后修复的源动力,研究发现,MyoD和Myf5可以驱动细胞周期进程的相关基因转录,伴随着配对盒转录因子Pax7下调,诱导细胞周期退出,并上调肌生成素和肌源性调节因子4的表达,从而分化并融合形成新的再生的肌纤维[11]。另外,在正常静止状态的卫星细胞中,MyoD的表达无法检测,相反,骨骼肌处于运动或者肌肉损伤状态时,MyoD会被激活并调控成肌细胞细胞周期的退出,促进成肌细胞增殖与分化[13-14]。也有研究发现,缺少MyoD和Myf5的卫星细胞在未受伤的肌肉中仍然得以维持,但在受伤的肌肉中恰恰相反[15-16]。这提示在肌肉再生方面MyoD和Myf5是起着决定性作用的,并且其在肌肉损伤后强烈表达,并赋予肌肉分化的潜能。此外,通过敲除MyoD基因发现,小鼠骨骼肌并没有表现出明显的发育缺陷。但在Myf5突变的小鼠中,发现肌分化因子与肌生成素表达水平正常,骨骼肌也并没有表现出明显的发育缺陷,所以MyoD与Myf5的激活与表达可能是相互独立并具有一定的功能协同性,MyoD在一定程度上也可以取代Myf5在骨骼肌发育过程中的缺失,如果两者均表达缺失,则会表现出明显的骨骼肌发育缺失[11]。 除此之外,肌生成素和肌源性调节因子4参与单核成肌细胞融合为多核成肌细胞,最终形成成熟的肌管及维持肌纤维稳态发挥重要作用[13,17-18]。研究发现,通过敲除肌源性调节因子4发现,大鼠肌纤维出现肥大状态,蛋白质合成增加,进而提高肌细胞增强因子2(MEF2)表达水平,影响骨骼肌正常生长[12]。同时,也有研究显示, 肌生成素的缺失会阻止肌细胞融合并导致肌纤维数量减少,进而无法维持肌纤维稳态[19]。因此,生肌调节因子家族不仅可以调控自身基因表达,同时也能够相互补偿并调节相关靶基因的表达,促进成肌细胞增殖与分化,从而提高骨骼肌损伤的恢复质量。 2.2.2 视网膜母细胞瘤家族 视网膜母细胞瘤家族(Retinoblastoma,Rb)又称口袋蛋白,主要包括pRb、p130、p107,其主要以磷酸化和非磷酸化两种形式存在[20]。据报道,这些蛋白质与MyoD家族协同介导肌细胞从细胞周期退出,并调控肌细胞调节因子的表达来实现终末分化,进而促进骨骼肌发生[21-22]。研究发现,pRb能够显著上调肌分化因子表达水平,并诱导肌肉分化标志物肌素重链和肌肉肌酸激酶的表达[23],也能够提高肌细胞增强因子2C的转录功能及活性,使细胞周期永久退出,进一步增强后期肌肉分化标志物表达,如肌源性调节因子4和肌肉肌酸激酶,从而促进成肌细胞分化[24-25]。而肌肉缺失pRb时,p107或p130代替pRb发挥作用,增加MyoD转录活性,上调肌素重链和肌肉肌酸激酶的表达,进一步表明pRb介导肌源因子表达和诱导细胞周期退出,促进肌生成[26]。除此之外,也有研究显示,pRb和p130对肌细胞分化起着正向调节作用,过度表达p130或pRb则会阻止细胞周期退出,进而抑制肌细胞分化,但p130仍可保留肌细胞的储备能力[27-28]。因此,视网膜母细胞瘤家族可以提高骨骼肌成肌细胞增殖与分化,其机制可能与MyoD家族协同作用有关,见图4。 "

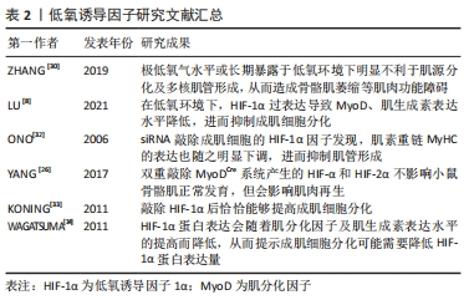
2.2.3 低氧诱导因子 氧水平对肌卫星细胞的静止、增殖、分化和自我更新存在密切联系。研究表明,长时间持续缺氧会降低MyoD和肌生成素表达水平,抑制肌细胞分化,进而影响骨骼肌发育及损伤后修复的过程[29]。ZHANG等[30]也进一步证明了这一观点,发现极低氧气水平或长期暴露于低氧环境下明显不利于肌源分化及多核肌管形成,从而造成骨骼肌萎缩等肌肉功能障碍。 低氧诱导因子(hypoxia inducible fators,HIF)是一种调控组织细胞氧稳态的关键性转录因子,主要包括HIF-1α、HIF-2α、HIF-3α,精确维持肌肉生长与再生之间的平衡,其表达受到氧浓度的调控,发挥着调控细胞能量代谢、细胞增殖与分化等生物效应[31]。有研究发现,在低氧环境下,HIF-1α过表达导致肌分化因子和肌生成素表达水平降低,进而抑制成肌细胞分化[8]。此外,有研究通过siRNA敲除成肌细胞的HIF-1α,发现肌素重链的表达也随之明显下调,进而抑制肌管形成,这说明低氧诱导因子在体内肌肉再生组织中起着重要作用,且是成肌细胞体外分化所必需的[32]。 但迄今为止,低氧诱导因子在成肌细胞中的作用仍存在争议,如低氧诱导因子的过表达、减少甚至敲除是否会影响成肌细胞增殖与分化的功能。通过双重敲除MyoDCre系统产生的HIF-1α和HIF-2α不影响小鼠骨骼肌正常发育,但会影响肌肉再生。然而,利用他莫昔芬诱导肌卫星细胞特异性Pax7CrER系统产生的HIF-1α/2αDko则会减少肌细胞数量,但不影响成肌细胞增殖与分化[26]。相反的是,敲除HIF-1α后恰恰能够提高成肌细胞分化[33]。然而,有研究显示,在诱导分化时HIF-1α蛋白表达会随着MyoD及肌生成素表达水平的提高而降低,从而提示成肌细胞分化可能需要降低HIF-1α蛋白表达量[34]。这些结果说明了低氧诱导因子的过表达、减少甚至敲除对成肌细胞增殖与分化的功能还需要进一步探究,但适量的HIF-1α可能促进成肌细胞分化来诱导肌生成,但过量的HIF-1α可能不是肌源性分化所必要的,见表2。 "
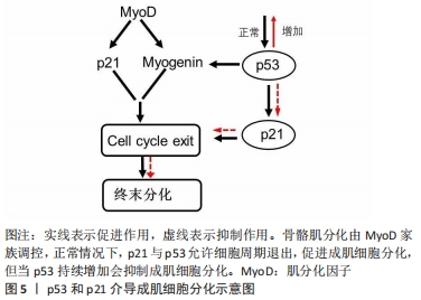
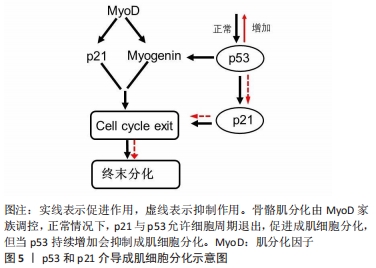
(2)细胞周期抑制性蛋白p21:p21蛋白是一种细胞周期抑制性蛋白,是细胞周期退出的关键调节器[38]。体内外实验表明,在肌细胞启动分化时,生肌调节因子MyoD上调细胞周期抑制性蛋白 p21的表达,进而抑制细胞周期蛋白依赖性激酶的活性,影响肌细胞在G1期的逆转过程,同时增强视网膜母细胞瘤蛋白的磷酸化,从而影响肌细胞分化过程中的细胞周期退出,导致肌细胞分化异常[39]。进一步研究表明,细胞周期抑制性蛋白p21表达的减少可以促进肌细胞增殖,反之,若通过抑制肌生成素和肌球蛋白重链4等肌源基因的转录则会抑制细胞周期抑制性蛋白p21在成肌细胞分化中的作用[40]。SUN等[41]研究发现,Akirin19(一种蛋白编码基因)异位表达可以上调P38MAPK和PI3K通路使p21的表达增加,进而诱导肌源性调节因子4、肌细胞增强因子2B增加,引起细胞周期退出,进一步促进肌细胞分化。因此,细胞周期抑制性蛋白p21可以通过调控细胞周期退出,促进成肌细胞分化,进一步阐明细胞周期抑制性蛋白p21调节肌细胞形成及再生的分子学机制,见图5。 "

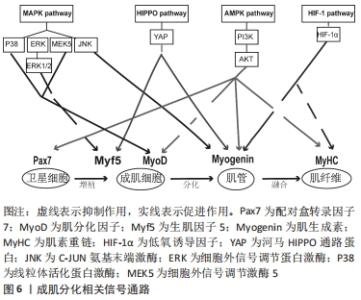
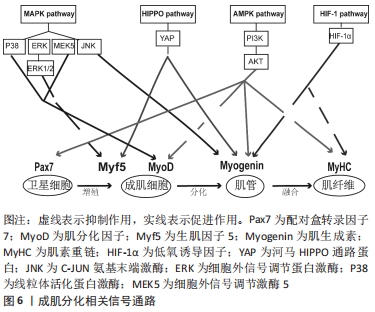
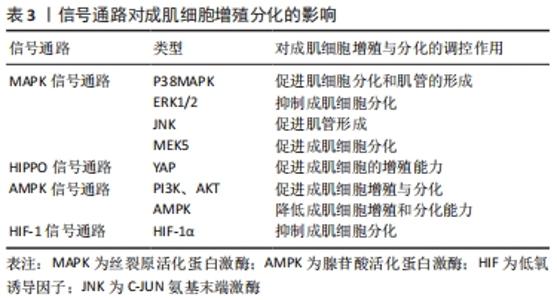
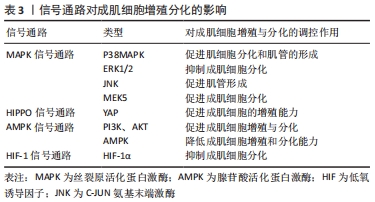
2.3.1 丝裂原活化蛋白激酶(mitogen-activated protein kinase,MAPK)信号通路 MAPK信号通路是调节成肌细胞增殖与分化的关键转导途径,在成肌细胞生长发育及损伤修复扮演着非常重要的角色[42],其主要包括C-JUN氨基末端激酶(C-Jun N-terminal kinase,JNK)、细胞外信号调节蛋白激酶(extracellular regulated kinase,ERK)和线粒体活化蛋白激酶(P38MAPK)以及ERK5[43]。CHEN等[44]研究表明,在成肌细胞分化过程中,通过激活MAPK/ERK激酶5和ERK5会促进成肌细胞分化,相反,ERK激酶5抑制剂的加入能够显著降低ERK5的表达,破坏ERK5/ERK激酶5通路,从而抑制成肌细胞分化。也有证据表明,提高P38MAPK信号通路磷酸化,能够激活肌分化因子,肌素重链转录因子的关键作用,促进肌细胞分化和肌管的形成,进而促进骨骼肌再生与修复[42]。此外,研究显示,在骨骼肌发育和再生过程中,通过抑制P38MAPK磷酸化,进而激活ERK1/2和AKT信号通路,降低肌素重链、肌分化因子和肌生成素的表达水平,导致细胞周期无法退出,进而抑制成肌细胞分化[45]。另外,进一步研究发现,P38MAPK信号通路的激活会增强MyoD和E蛋白的异质化,诱导肌细胞分化,从而进一步改善受损肌肉的再生能力[46]。这些发现都提示MAPK信号通路能够调控成肌细胞分化,对于骨骼肌再生是必不可少的。 2.3.2 河马HIPPO信号通路 河马HIPPO信号转导通路已被证实在成肌细胞增殖与分化中发挥关键作用。当HIPPO通路的转录因子YAP的活性和表达水平升高时,激活成肌细胞肌源因子表达,如生肌调节因子Myf6、骨形态发生蛋白4(BMP4)、跨膜糖蛋白(CD34),进而促进成肌细胞的增殖能力。相反,敲除YAP的表达会降低成肌细胞增殖数量,进而影响肌细胞发育[47-48]。这提示YAP表达量增加可参与调节成肌细胞增殖,为骨骼肌损伤后的修复提供稳定的肌卫星细胞储备,有助于骨骼肌功能恢复。有研究证明,通过过表达YAP蛋白突变体(HYAP1 S127A)会降低肌素重链及细胞周期抑制性蛋白P21的表达、上调Myf5的表达,诱导未磷酸化的YAP的表达量增多,进而影响细胞周期退出,使成肌细胞无法分化为肌管,不利于骨骼肌修复[49]。另外,KNYAZEVA等[50]发现抑制菲素C(Filamin C)的表达则会导致HIPPO信号通路失调,肌生成素表达降低,进而丧失肌源分化能力。上述结果说明通过调控HIPPO信号通路及其关键因子可以调节成肌细胞增殖与分化,从而为治疗骨骼肌损伤及修复提供理论依据。 2.3.3 腺苷酸活化蛋白激酶(AMP-activated protein kinase,AMPK)信号通路 AMPK信号通路作为细胞内能量代谢感受器和关键调节分子,在调节成肌细胞增殖与分化、肌肉再生中发挥着重要作用。有研究通过敲除AMPK信号转导通路发现,肌管阶段5-羟甲基胞嘧啶(5-HMC)水平持续下降,Pax7表达完全丧失,最终抑制成肌细胞分化能力[51]。此外,研究进一步发现,二甲双胍能够激活成肌细胞内AMPK信号通路,抑制细胞增殖,进而上调Pax7蛋白表达及降低肌分化因子表达水平,从而导致肌细胞维持低分化状态[7]。并且在成肌细胞分化过程中,过度激活AMPK信号通路会转变细胞周期进程,降低肌素重链和细胞周期抑制性蛋白p21的表达,进而影响肌管形成[5],说明在成肌细胞中腺苷酸活化蛋白激酶AMPK的过度激活和敲除会导致肌细胞增殖和分化能力下降,从而给骨骼肌的健康带来负面影响,并最终影响肌肉再生能力。 另外,在生理条件下,AMPK下游分子磷脂酰肌醇3激酶(phosphoinositide 3-kinase, PI3K)在细胞内表达较少,当细胞损伤时,会激活细胞因子、生长因子等细胞外信号分子,如G蛋白偶联受体(GPCR)、络氨酸激酶(RTK),使得PI3K表达迅速提高,调控下游信号分子AKT,进而促进成肌细胞增殖与分化[52]。通过研究骨骼肌损伤第3,5天发现,损伤组的PI3K、AKT、mTOR、P-mTORSer2448、P-AktSer473的蛋白表达量先升高后降低,同时MyoD和肌生成素表达水平先上调后下调;然后,运用苏木精-伊红染色发现骨骼肌损伤后会出现大量的新生的成肌细胞和肌管,且PI3K和AKT的表达水平与促成肌作用的MyoD和肌生成素的mRNA趋势保持一致,这表明该信号通路的激活可以调节成肌细胞增殖与分化,进而促进骨骼肌损伤修复[13,52]。不仅如此,也有研究发现,应用药物修复骨骼肌萎缩,其主要通过提高p110催化亚基的PI3K的磷酸化水平,激活PI3K/AKT表达,增加PI3K和AKT蛋白表达水平,从而促进成肌细胞分化[53]。以上这些研究说明成肌细胞增殖与分化与AMPK信号通路有关。 2.3.4 HIF-1信号通路 HIF-1α作为成肌细胞适应低氧环境重要的调节因子,HIF-1α的表达会介导HIF-1信号通路中的作用,进而影响成肌细胞增殖、分化功能。有研究显示,通过HIF-1α因子在成肌细胞中激活HIF-1信号通路,可以增强Notch活性抑制肌生成[54]。而最新研究发现,HIF-1信号通路可能与其他信号通路协同来调节成肌细胞增殖与分化,如Wnt、AMPK。MAJMUNDAR 等[54]发现HIF-1α也可能通过抑制Wnt信号来抑制成肌细胞分化,进而抑制肌生成。另外,HIF-1α也可以通过抑制AMPK通路在肌细胞分化中的作用,进而损害成肌细胞结构,抑制线粒体生物合成,从而抑制成肌细胞分化[8]。所以,通过介导低氧诱导因子激活HIF-1信号通路能够调节处于低氧环境的成肌细胞增殖与分化能力,维持骨骼肌稳态,且该通路也会协同其他信号通路来抑制成肌细胞分化,这些均表明HIF-1作为转录因子及信号通路在成肌细胞分化过程中发挥重要作用。 "

| [1] OLGUIN HC, OLWIN BB. Pax-7 up-regulation inhibits myogenesis and cell cycle progression in satellite cells: a potential mechanism for self-renewal. Dev Biol. 2004;275(2):375-388. [2] XIONG G, HINDI SM, MANN AK, et al. The PERK arm of the unfolded protein response regulates satellite cell-mediated skeletal muscle regeneration. Elife. 2017;6:e22871. [3] GUITART M, LLORETA J, MAÑAS-GARCIA L, et al. Muscle regeneration potential and satellite cell activation profile during recovery following hindlimb immobilization in mice. J Cell Physiol. 2018;233(5):4360-4372. [4] ZHANG J, XU X, LIU Y, et al. EPA and DHA Inhibit Myogenesis and Downregulate the Expression of Muscle-related Genes in C2C12 Myoblasts. Genes (Basel). 2019;10(1):64. [5] 王震,蔺海旗,何霏,等.运动激活骨骼肌卫星细胞:增龄性肌衰减症及肌肉损伤修复的运动预防和治疗[J].中国组织工程研究, 2021,25(23):3752-3759. [6] 周静,郑孝众,姚婉贞,等.骨骼肌运动损伤的MRI研究进展[J].中华放射学杂志,2021,55(3):324-328. [7] 蒲荣喜. AMPK对成肌细胞功能活性的影响及其代谢调节研究[D].重庆:第三军医大学,2017. [8] LU Y, MAO J, HAN X, et al. Downregulated hypoxia-inducible factor 1α improves myoblast differentiation under hypoxic condition in mouse genioglossus. Mol Cell Biochem. 2021;476(3):1351-1364. [9] DUMONT NA, RUDNICKI MA. Characterizing Satellite Cells and Myogenic Progenitors During Skeletal Muscle Regeneration. Methods Mol Biol. 2017;1560:179-188. [10] 从晓霞. mTOR信号通路在肌肉再生和肌腱分化中的作用和调控机制研究[D].杭州:浙江大学,2018. [11] HERNÁNDEZ-HERNÁNDEZ JM, GARCÍA-GONZÁLEZ EG, BRUN CE, et al. The myogenic regulatory factors, determinants of muscle development, cell identity and regeneration. Semin Cell Dev Biol. 2017;72:10-18. [12] ZAMMIT PS. Function of the myogenic regulatory factors Myf5, MyoD, Myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis. Semin Cell Dev Biol. 2017;72:19-32. [13] 张安宁,罗雪林,黄思琴,等.PI3K/Akt/mTOR信号通路在大鼠急性骨骼肌钝挫伤修复中的作用[J].中国运动医学杂志,2018,37(7):594-600. [14] JUNG EM, JEUNG EB. Rapamycin-induced au-tophagy decreases Myf5 and MyoD proteins in C2C1myoblast cells. Toxicol In Vitro. 2019;58: 132-141. [15] YAMAMOTO M, LEGENDRE NP, BISWAS AA, et al. Loss of MyoD and Myf5 in Skeletal Muscle Stem Cells Results in Altered Myogenic Programming and Failed Regeneration. Stem Cell Reports. 2018;10(3): 956-969. [16] ZAPPIA MP, ROGERS A, ABMMK I, et al. Rbf Activates the Myogenic Transcriptional Program to Promote Skeletal Muscle Differentiation. Cell Rep. 2019;26(3):702-719.e6. [17] ASFOUR HA, ALLOUH MZ, SAID RS. Myogenic regulatory factors: The orchestrators of myogenesis after 30 years of discovery. Exp Biol Med (Maywood). 2018;243(2):118-128. [18] 汪瑞婷.骨骼肌特异转录调控因子MyoD通过相分离机制调控基因表达[D].北京:北京协和医学院,2019. [19] YANG ZJ, BROZ DK, NODERER WL, et al. p53 suppresses muscle differentiation at the myogenin step in response to genotoxic stress. Cell Death Differ. 2015;22(4):560-573. [20] MASCIULLO V, VALDIVIESO P, AMADIO G, et al. Role of Retinoblastoma Protein Family (Rb/p105 and Rb2/p130) Expression in the Histopathological Classification of Borderline Ovarian Tumors. Front Med (Lausanne). 2020;7:596226. [21] SCHNEIDER JW, GU W, ZHU L, et al. Reversal of terminal differentiation mediated by p107 in Rb-/- muscle cells. Science. 1994;264(5164):1467-1471. [22] SIDLE A, PALATY C, DIRKS P, et al. Activity of the retinoblastoma family proteins, pRB, p107, and p130, during cellular proliferation and differentiation. Crit Rev Biochem Mol Biol. 1996;31(3):237-271. [23] DE FALCO G, COMES F, SIMONE C. pRb: master of differentiation. Coupling irreversible cell cycle withdrawal with induction of muscle-specific transcription. Oncogene. 2006;25(38):5244-5249. [24] NOVITCH BG, SPICER DB, KIM PS ,et al. pRb is required for MEF2-dependent gene expression as well as cell-cycle arrest during skeletal muscle differentiation. Curr Biol. 1999;9(9):449-459. [25] ROUFAYEL R, MEZHER R, STOREY KB. The Role of Retinoblastoma Protein in Cell Cycle Regulation: An Updated Review. Curr Mol Med. 2021;21(8):620-629. [26] YANG X, YANG S, WANG C, et al. The hypoxia-inducible factors HIF1α and HIF2α are dispensable for embryonic muscle development but essential for postnatal muscle regeneration. J Biol Chem. 2017;292(14): 5981-5991. [27] GENOVESE C, TRANI D, CAPUTI M, et al. Cell cycle control and beyond: emerging roles for the retinoblastoma gene family. Oncogene. 2006; 25(38):5201-5209. [28] CARNAC G, FAJAS L, L’HONORÉ A, et al. The retinoblastoma-like protein p130 is involved in the determination of reserve cells in differentiating myoblasts. Curr Biol. 2000;10(9):543-546. [29] WANG C, LIU W, LIU Z, et al. Hypoxia Inhibits Myogenic Differentiation through p53 Protein-dependent Induction of Bhlhe40 Protein. J Biol Chem. 2015;290(50):29707-29716. [30] ZHANG Z, ZHANG L, ZHOU Y, et al. Increase in HDAC9 suppresses myoblast differentiation via epigenetic regulation of autophagy in hypoxia. Cell Death Dis. 2019;10(8):552. [31] 宋亚琼,周播江.低氧诱导因子-1在调控骨骼肌缺氧时能量代谢发生适应性变化的机制研究进展[J].解剖学报,2017,48(2):236-240. [32] ONO Y, SENSUI H, SAKAMOTO Y, et al. Knockdown of hypoxia-inducible factor-1alpha by siRNA inhibits C2C12 myoblast differentiation. J Cell Biochem. 2006;98(3):642-649. [33] KONING M, WERKER PM, VAN LUYN MJ, et al. Hypoxia promotes proliferation of human myogenic satellite cells: a potential benefactor in tissue engineering of skeletal muscle. Tissue Eng Part A. 2011;17(13-14): 1747-1758. [34] WAGATSUMA A, KOTAKE N, YAMADA S. Spatial and temporal expression of hypoxia-inducible factor-1α during myogenesis in vivo and in vitro. Mol Cell Biochem. 2011;347(1-2):145-155. [35] LIU X, WANG Y, ZHAO S, et al. Fibroblast Growth Factor 21 Promotes C2C12 Cells Myogenic Differentiation by Enhancing Cell Cycle Exit. Biomed Res Int. 2017;2017:1648715. [36] FLAMINI V, GHADIALI RS, ANTCZAK P, et al. The Satellite Cell Niche Regulates the Balance between Myoblast Differentiation and Self-Renewal via p53. Stem Cell Reports. 2018;10(3):970-983. [37] LI H, HOU L, ZHANG Y, et al. PFN2a Suppresses C2C12 Myogenic Development by Inhibiting Proliferation and Promoting Apoptosis via the p53 Pathway. Cells. 2019;8(9):959. [38] ADHIKARI A, MAINALI P, DAVIE JK. JARID2 and the PRC2 complex regulate the cell cycle in skeletal muscle. J Biol Chem. 2019;294(51): 19451-19464. [39] MAL A, CHATTOPADHYAY D, GHOSH MK, et al. p21 and retinoblastoma protein control the absence of DNA replication in terminally differentiated muscle cells. J Cell Biol. 2000;149(2):281-292. [40] WANG S, ZUO H, JIN J, et al. Long noncoding RNA Neat1 modulates myogenesis by recruiting Ezh2. Cell Death Dis. 2019;10(7):505. [41] SUN W, HU S, HU J, et al. Akirin1 promotes myoblast differentiation by modulating multiple myoblast differentiation factors. Biosci Rep. 2019;39(3):BSR20182152. [42] GUO Y, WANG M, GE J, et al. Bioactive biodegradable polycitrate nanoclusters enhances the myoblast differentiation and in vivo skeletal muscle regeneration via p38 MAPK signaling pathway. Bioact Mater. 2020;5(3):486-495. [43] SUN Y, LIU WZ, LIU T, et al. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J Recept Signal Transduct Res. 2015;35(6):600-604. [44] CHEN TH, CHEN CY, WEN HC, et al. YAP promotes myogenic differentiation via the MEK5-ERK5 pathway. FASEB J. 2017;31(7):2963-2972. [45] CONTRERAS O, VILLARREAL M, BRANDAN E. Nilotinib impairs skeletal myogenesis by increasing myoblast proliferation. Skelet Muscle. 2018; 8(1):5. [46] YOO M, LEE SJ, KIM YK, et al. Dehydrocorydaline promotes myogenic differentiation via p38 MAPK activation. Mol Med Rep. 2016;14(4): 3029-3036. [47] JUDSON RN, TREMBLAY AM, KNOPP P, et al. The Hippo pathway member Yap plays a key role in influencing fate decisions in muscle satellite cells. J Cell Sci. 2012;125(Pt 24):6009-6019. [48] 赵莉娟,张宏,张国辉,等.Hippo信号通路在骨骼肌损伤修复中作用的研究进展[J].山东医药,2020,60(1):109-112. [49] WATT KI, JUDSON R, MEDLOW P, et al. Yap is a novel regulator of C2C12 myogenesis. Biochem Biophys Res Commun. 2010;393(4):619-624. [50] KNYAZEVA A, KHUDIAKOV A, VAZ R, et al. FLNC Expression Level Influences the Activity of TEAD-YAP/TAZ Signaling. Genes (Basel). 2020; 11(11):1343. [51] ZHANG T, GUAN X, CHOI UL, et al. Phosphorylation of TET2 by AMPK is indispensable in myogenic differentiation. Epigenetics Chromatin. 2019;12(1):32. [52] 吕欣,周达岸.PI3K/AKT信号通路对骨骼肌再生的影响研究进展[J].中国运动医学杂志,2020,39(11):908-912. [53] ZHANG ZK, LI J, LIU J, et al. Icaritin requires Phosphatidylinositol 3 kinase (PI3K)/Akt signaling to counteract skeletal muscle atrophy following mechanical unloading. Sci Rep. 2016;6:20300. [54] MAJMUNDAR AJ, LEE DS, SKULI N, et al. HIF modulation of Wnt signaling regulates skeletal myogenesis in vivo. Development. 2015; 142(14):2405-2412. |
| [1] | He Xi, Wan Yu, Tang Yuting, Yang Anning, Wu Kai, Jiao Yun, Bai Zhigang, Jiang Yideng, Shen Jiangyong. Erastin inhibits proliferation of hypertrophic scar fibroblasts [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(在线): 1-. |
| [2] | Yang Zhishan, Tang Zhenglong. YAP/TAZ, a core factor of the Hippo signaling pathway, is involved in bone formation [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1264-1271. |
| [3] | Zhao Lu, Zhao Yifei, Gao Da, Liu Yanfang, Fu Tingting, Xu Jiangyan. Expression of suppressor of Zeste 12 in kidney tissues of rats with diabetic nephropathy [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1179-1186. |
| [4] | Zhang Yan, He Ruibo, Wang Qingbo, Pi Yihua, Lu Chunmin, Xu Chuanyi, Ma Gang, Peng Peng. Effects of aerobic exercises with different load volumes on inflammatory response and insulin signaling pathway of skeletal muscle in obese rats [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1237-1244. |
| [5] | Liu Xiaolin, Mu Xinyue, Ma Ziyu, Liu Shutai, Wang Wenlong, Han Xiaoqian, Dong Zhiheng. Effect of hydrogel-loaded simvastatin microspheres on osteoblast proliferation and differentiation [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7): 998-1003. |
| [6] | Liu Wentao, Feng Xingchao, Yang Yi, Bai Shengbin. Effect of M2 macrophage-derived exosomes on osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 840-845. |
| [7] | Long Yanming, Xie Mengsheng, Huang Jiajie, Xue Wenli, Rong Hui, Li Xiaojie. Casein kinase 2-interaction protein-1 regulates the osteogenic ability of bone marrow mesenchymal stem cells in osteoporosis rats [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 878-882. |
| [8] | Li Xinyue, Li Xiheng, Mao Tianjiao, Tang Liang, Li Jiang. Three-dimensional culture affects morphology, activity and osteogenic differentiation of human periodontal ligament stem cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 846-852. |
| [9] | Li Qicheng, Deng Jin, Fu Xiaoyang, Han Na. Effects of bone marrow mesenchymal stem cells-derived exosomes on hypoxia-treated myoblasts [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 853-859. |
| [10] | Yuan Wei, Liu Jingdong, Xu Guanghui, Kang Jian, Li Fuping, Wang Yingjie, Zhi Zhongzheng, Li Guanwu. Osteogenic differentiation of human perivascular stem cells and its regulation based on Wnt/beta-catenin signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 866-871. |
| [11] | Qiao Luhui, Ma Ziyu, Guo Haoyu, Hou Yudong. Comparison of puerarin and icariin on the biological properties of mouse preosteoblasts [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 872-877. |
| [12] | Hao Liufang, Duan Hongmei, Wang Zijue, Hao Fei, Hao Peng, Zhao Wen, Gao Yudan, Yang Zhaoyang, Li Xiaoguang. Spatiotemporal dynamic changes of ependymal cells after spinal cord injury in transgenic mice [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 883-889. |
| [13] | Yuan Bo, Xie Lide, Fu Xiumei. Schwann cell-derived exosomes promote the repair and regeneration of injured peripheral nerves [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 935-940. |
| [14] | Liu Hongwen, Li Jiao, Xu Wenhao, Nie Hua, Liu Shaojiang, Xu Jie, Yin Li. Differential expression profiles of microRNAs in muscle tissue of denervated skeletal muscle atrophy rats [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(5): 732-737. |
| [15] | Li Long, Li Guangdi, Shi Hao, Deng Keqi. Circular RNA as a competing endogenous RNA is involved in the regulation of osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(5): 751-757. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||