Chinese Journal of Tissue Engineering Research ›› 2022, Vol. 26 ›› Issue (25): 4058-4063.doi: 10.12307/2022.414
Previous Articles Next Articles
Mesenchymal stem cells promote wound healing by regulating the autophagy
Zhang Gaofei, Wang Di, Li Jiamei, Lou Hanxiao, Zeng Yueqin, Liu Wenjun
- Department of Burns, Second Affiliated Hospital of Kunming Medical University, Kunming 650188, Yunnan Province, China
-
Received:2021-03-12Accepted:2021-05-28Online:2022-09-08Published:2022-01-26 -
Contact:Liu Wenjun, MD, Associate professor, Department of Burns, Second Affiliated Hospital of Kunming Medical University, Kunming 650188, Yunnan Province, China -
About author:Zhang Gaofei, Master, Department of Burns, Second Affiliated Hospital of Kunming Medical University, Kunming 650188, Yunnan Province, China -
Supported by:National Natural Science Foundation of China, No. 82060349 (to LWJ); Graduate Innovation Foundation, No. 2020S182 (to ZGF); Kunming Medical Joint Special Project, No. 2018FE001(-242) (to LWJ)
CLC Number:
Cite this article
Zhang Gaofei, Wang Di, Li Jiamei, Lou Hanxiao, Zeng Yueqin, Liu Wenjun. Mesenchymal stem cells promote wound healing by regulating the autophagy[J]. Chinese Journal of Tissue Engineering Research, 2022, 26(25): 4058-4063.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
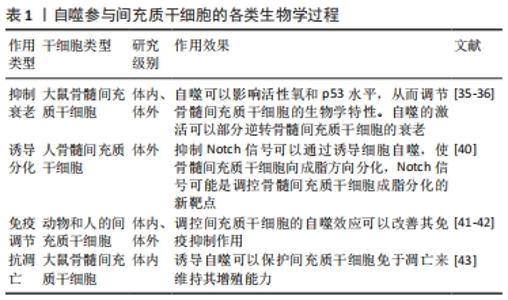
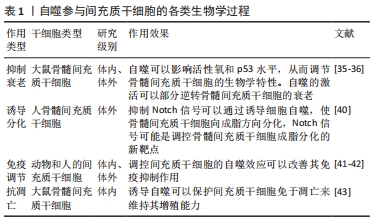
2.1 间充质干细胞促进创面愈合的作用机制 创面愈合是涉及一系列复杂生物学行为的动态过程,其中包括应激信号的激活和传递、炎症反应的失衡以及各种有害因子的释放等。创面修复过程主要基于修复细胞、炎性细胞、细胞外基质和生长因子的协同作用,共同对受损的软组织进行修复重建[14]。 创面修复主要分为3个阶段:炎症期、细胞增殖期与组织重塑期,烧伤创面在这3个时期的愈合过程中需要多种细胞以及相关的细胞因子乃至细胞外基质等共同参与[15]。间充质干细胞的支持性和调节旁分泌的特性在皮肤创面修复中至关重要[16]。越来越多的证据表明,间充质干细胞在治疗各种炎症性疾病、自身免疫性疾病和变性疾病方面显示出优异的治疗效果[17-19]。此外,间充质干细胞还能够促进同种异体移植皮肤的长期存活[20]。间充质干细胞的自我更新和多系分化能力以及强大的免疫调控功能被认为是其治疗创面的基础,将间充质干细胞注射入体内,它们便能够通过分化为特定的细胞谱系来介导受损组织的修复,此外,间充质干细胞还能够通过分泌可溶性细胞因子和膜结合分子或通过与免疫细胞的相互作用来调节免疫反应[21-22]。现有数据表明,间充质干细胞移植后会受到局部微环境的刺激,它们能够产生一系列参与许多生物学过程的生物活性物质,这些生物学过程包括免疫调节[22]、血管生成[23]、抗凋亡[24]、抗氧化[25]、抗纤维化[26]。间充质干细胞的这些功能对于创面治疗具有积极的作用。据报道,间充质干细胞旁分泌的众多生长因子不仅可以促进创面血管生成,而且可以动员创面和创周的干细胞、真皮成纤维细胞、内皮细胞以及角质细胞的活化、迁移、增殖和分泌胶原蛋白,共同促进创面愈合[27-28]。研究表明,与假手术相比,采取间充质干细胞局部处理的小鼠成纤维细胞的增殖能力明显增强[29]。进一步研究发现,间充质干细胞能够促进烧伤后创面组织细胞的增殖、胶原沉积以及表皮再上皮化从而促进烧伤创面的修复[30-31]。此外,间充质干细胞还促进与伤口愈合有关的多种类型细胞向损伤部位迁移,包括成纤维细胞和角质形成细胞[32],这些结果说明间充质干细胞可以促进创面愈合,但具体机制尚未完全阐明。 虽然间充质干细胞具有自我更新能力,但随着细胞培养时间的延长,细胞逐渐衰变、老化,分化潜能逐渐降低,而自噬对干细胞命运的决策具有重要影响[33]。自噬是细胞中高度保守的物质降解过程,当自噬体形成时,它会包裹住某些细胞成分,例如那些被破坏的蛋白质和细胞器.最终自噬体与溶酶体融合,这些细胞成分便会降解为更小成分,这一过程为细胞的更新提供了养分和构建基础,细胞自噬有着广泛的生理和病理意义 [34]。研究表明,自噬的激活可以部分逆转间充质干细胞衰老[35-36],提示自噬对于维持间充质干细胞生物学功能起着至关重要的作用[37]。间充质干细胞移植后能通过上调细胞自噬水平促进肝细胞再生[38]。Notch信号和自噬信号途径之间既相互交织在一起,又可以被其他机制同时影响从而促进细胞间的相互作用[39]。抑制Notch信号可以通过PTEN-PI3K/Akt/mTOR诱导细胞自噬,使骨髓间充质干细胞向成脂方向分化[40]。此外,间充质干细胞移植前通过细胞外刺激调节自噬不仅可以影响细胞分化、存活和衰老,还可以增强间充质干细胞的免疫调节作用[41]。研究表明,雷帕霉素通过抑制mTOR信号传导途径改善了间充质干细胞的免疫抑制作用[42]。WANG等[43]发现低浓度地塞米松处理可通过诱导自噬并保护细胞免于凋亡来维持间充质干细胞的增殖能力,而高浓度的地塞米松会诱发明显的细胞毒性,见表1。"

XIAO等[44]研究表明,在烧伤创面早期观察到自噬效应和创面血流量减少以及炎症增加。此外,多项研究发现自噬可以通过直接抑制炎症复合物和间接消除炎症刺激来抑制炎症反应[45-47],同时局部组织缺氧或者组织低灌注等也可诱导自噬,促进创面细胞存活及血管新生[48-49]。在胶原重塑期,自噬还可以通过不同信号通路参与调控纤维化过程中的细胞代谢[50]。据报道,自噬相关基因Beclin-1在烧伤创面的表达量显著增加,并发现增加自噬有助于创面愈合[51]。XU等[52]研究提示诱导自噬可以增强铜绿假单胞菌在糖尿病伤口中的清除。雷公藤甲素可通过抑制PI3K/AKT/mTOR信号通路进而抑制成纤维细胞增殖,刺激细胞凋亡和自噬性死亡而发挥抗纤维化作用[53]。角质形成细胞的迁移受p38MAPK/自噬信号通路的调控,激活p38MAPK/自噬通路可促进角质形成细胞的迁移[54]。这些研究都提示间充质干细胞可能是通过调控自身的自噬效应从而发挥了强大的创面治疗作用。 2.2 自噬的分类及作用机制 根据自噬生理功能和传递到溶酶体的方式不同,已经确定了3种形式的自噬——伴侣自噬、微自噬和巨自噬[55]。巨自噬(以下简称自噬)指的是降解细胞内成分的过程,包括细胞质、一些细胞器和细胞膜、蛋白质和核酸等。自噬参与细胞以及生物体的发育和分化的全过程,与细胞的增殖以及损伤后的修复等具有密切关系。目前的观点认为,自噬的主要功能之一是细胞在受到应激性死亡威胁时仍然能够保持细胞的存活[56-57]。 DE DUVE等[58]创造了“自噬”一词,描述的是具有单层或双层膜的结构吞噬受损的蛋白质和细胞器等细胞组分形成自噬体,之后与溶酶体融合形成自噬溶酶体,最后将包裹物降解的一个过程。1973年,BOLENDER和WEIBEL[59]首次提供了一些证据,证明一种特定的细胞器(光滑内质网)可以被自体吞噬,后来LEMASTERS和同事发现线粒体膜电位的变化导致了自噬的发生。此外,无论是酵母还是高等的真核生物细胞中均存在许多类型的选择性自噬[60]。 自噬参与多种细胞功能的调控[56-57],日本生物学家大隅良典(YOSHINORI OHSUMI)因发现这种“自噬”机制而获得诺贝尔奖[61]。正常情况下,细胞自噬维持在较低水平以执行体内稳态功能,例如蛋白质和细胞器更新。营养状况、激素和其他因素(例如温度、氧气浓度和细胞密度)对于控制自噬至关重要。当细胞需要产生能量并进行结构重组时,自噬水平会迅速上调[62-64]。 完整的自噬过程包括噬菌体诱导、成核和扩增,自噬小体成熟和与液泡/溶酶体融合以及自噬物的分解和外排[65-67]。自噬的第一步,即自噬体的生物发生,依赖于由ATG(自噬相关)基因和ATG蛋白组成的核心机制[68]。首先,mTOR调控的信号转导通路受到刺激后启动自噬,在囊泡成核过程中,vps34-beclin-1和PI3KIII复合物介导分离膜(也称为吞噬细胞)的形成,atg5-atg12-atg16复合物和Atg8/LC3蛋白(经过Atg4处理以暴露其羧基末端甘氨酸残基的泛素样蛋白)调节延伸阶段,在该阶段吞噬细胞膜伸长形成双层囊泡,称为自噬体[69]。自噬体的形成是自噬过程中的一个标志性事件,ULK1复合物是自噬的关键启动因子,其活性主要通过mTORC1和AMPK在不同位点磷酸化来调节[70-71]。mTOR是保守的丝氨酸/苏氨酸蛋白激酶,属于PI3K相关的蛋白激酶家族[72]。mTOR是2种不同的多蛋白复合物的催化成分:mTOR复合物1 (mTORC1)和mTOR复合物2 (mTORC2)。mTORC1对雷帕霉素敏感,mTORC2则不敏感[73]。目前mTOR调控自噬的几个关键通路有mTOR/AMPK/ULK1信号通路、mTOR/vps34-atg14复合信号通路、mTOR/TFEB/ TFE3和自噬溶酶体信号通路(ALR)[74]。 unc51样激酶1 (ULK1)与ATG13、ATG101和200 kD的黏附激酶家族相互作用蛋白(FIP200)相互作用,组成ULK1复合物[75-76]。正常情况下,mTORC1磷酸化ULK1的P757位点,干扰AMPK和ULK1的相互作用,抑制自噬的启动。在营养缺乏或其他细胞胁迫下,ULK1从mTORC1中释放出来,而mTORC1被抑制,通过AMPK在多个位点磷酸化而被激活[77]。AMPK的这种磷酸化已被证明在大多数情况下可诱导自噬[78]。 2.3 间充质干细胞的自噬效应对创面愈合的影响 众所周知,创面愈合是一个动态和复杂的多相过程,涉及生长因子、细胞因子和各种细胞之间的协调相互作用,任何阶段失败都可能导致慢性创面形成和异常瘢痕增生[79]。研究表明,间充质干细胞可以保护血管基膜,使其免受基质金属蛋白酶的降解[80],并促进真皮成纤维细胞合成纤维连接蛋白、胶原蛋白和弹性蛋白,提示间充质干细胞具有促进创面愈合的潜力[81]。间充质干细胞直接或间接的分泌功能诱导成纤维细胞增殖、迁移和减少组织损伤,促进创面愈合[82]。此外有证据表明,间充质干细胞的促创面愈合作用是通过调控自噬引起的。研究显示,移植在受损创面中的间充质干细胞通过调节Becline-1来促进伤口成纤维细胞的增殖,进而促进创面愈合[83]。Becline-1在创面愈合中充当积极的调节器促进自噬,HIF-1α/BNIP3/Beclin-1被认为是在缺氧条件下自噬调控的重要信号途径[84]。RAMHORMOZI等[85]研究表明骨髓间充质干细胞与辛伐他汀结合可通过激活Akt/mTOR途径加速烧伤创面的愈合过程。WANG等[86]研究提示抑制骨髓间充质干细胞的AMPK/mTOR通路,可以抑制体内伤口愈合。因此,间充质干细胞移植创面后激活了间充质干细胞的自噬效应从而促进了创面的愈合过程。 2.3.1 间充质干细胞调控自噬促进创面成纤维细胞的增殖 成纤维细胞是肉芽组织的重要组成部分,也是创面愈合的主要物质。成纤维细胞是参与皮肤修复/瘢痕形成的最主要的细胞,其生物学效果对创面愈合起到了决定性的作用[87]。研究表明,雷帕霉素是哺乳动物mTOR信号通路的特异性负调节剂,雷帕霉素通过靶向mTORC1活化来激活自噬,从而调节受损创面成纤维细胞的增殖和迁移[88-89]。同时,雷帕霉素诱导的自噬在瘢痕疙瘩成纤维细胞中具有抗纤维化作用[90]。YOON等[91]通过实验结果得到瑞芬太尼预处理过氧化氢组通过PI3K信号通路上调ATG5、Becline-1、LC-3Ⅱ的表达,激活Akt-mTOR通路,进而激发人皮肤成纤维细胞对氧化应激的保护作用,促进成纤维细胞增殖,进而帮助创面修复。LI等[92]研究提示上调自噬相关蛋白Atg5和LC3-Ⅱ的表达,激活mTOR通路,成纤维细胞迁移速度显著提高。这些研究都表明自噬在创面愈合过程中十分重要,自噬效应能够影响创面成纤维细胞的增殖、分化和迁移,并与器官纤维化密切相关。 研究表明,转化生长因子β蛋白分泌相关的细胞因子参与创面肥厚性瘢痕形成[93]。转化生长因子β1是一种重要的促纤维化因子,可以诱导Ⅰ型胶原合成以及成纤维细胞分化,并促进成纤维细胞增殖[94]。转化生长因子β1可以通过Akt信号通路,进而调控纤维化的进程[95]。此外,转化生长因子β1还可以通过PI3K-Akt-mTOR及MAPK-细胞外信号调节激酶通路下调自噬,引起纤维细胞的过度增殖[96]。这些研究结果均提示转化生长因子β1可通过自噬效应来影响成纤维细胞的增殖和迁移。同时,转化生长因子β1也是 间充质干细胞分泌的关键细胞因子,其通过调节某些细胞因子的表达参与多种细胞的增殖和迁移。自噬作为一种细胞保护机制,在间充质干细胞发育过程中发挥着重要作用,间充质干细胞可以诱导自噬通过旁分泌转化生长因子β1负调节纤维化的发生发展过程[97]。研究表明,转化生长因子β1在间充质干细胞自噬激活中也起重要作用[98-99]。SUN等[100]研究表明转化生长因子β1通过加速间充质干细胞中的G1/S细胞周期转变来促进间充质干细胞增殖,证明转化生长因子β1可以激活Akt-mTOR-S6K1通路改变间充质干细胞的细胞周期,刺激间充质干细胞增殖。 间充质干细胞可有效促进创面成纤维细胞的增殖,提高创面愈合的速度和质量[101]。最新研究显示,创面损伤组织周围的微环境会影响间充质干细胞通过调控自噬进一步旁分泌生物活性因子加速损伤组织成纤维细胞的迁移,进而达到治疗目的[102-103]。史永平等[104]研究提示间充质干细胞通过调控其自噬效应旁分泌转化生长因子β1进而促进成纤维细胞的增殖和迁移,加速创面愈合。 2.3.2 间充质干细胞调控自噬改善创面的炎症反应 炎症期是创面愈合的第一阶段。大量的炎症细胞被激活,导致炎症细胞因子的失控性释放,进而导致创面发炎。在创面治疗过程中,减轻炎症对于预防组织损伤和纤维化很重要[105]。研究表明,间充质干细胞移植到烧伤创面后通过分泌细胞因子来抑制机体免疫细胞的过度激活,从而抑制创面过度炎症反应[106],其可能通过 Akt和wnt4/β-catenin 信号通路的激活来发挥创面修复作用的[107]。 XIE等[108]研究表明脐带间充质干细胞通过AKT/mTOR信号通路减轻炎症并抑制间质性膀胱炎的细胞凋亡。GAN等[109]研究表明间充质干细胞可以激活细胞的AMPK/mTOR依赖性自噬进而分泌白细胞介素6,从而抑制创面炎症反应。间充质干细胞还可以通过调控自噬效应治疗肠炎性小鼠的慢性炎症[110]。 2.3.3 间充质干细胞调控自噬促进创面血管生成 创面损伤通常与血管缺失有关,血管缺失会导致创面缺氧和活性氧积累引起氧化应激反应[111]。受损创面由于蛋白酶含量增多,导致创面新生血管以及肉芽组织生成不足,而间充质干细胞不仅可以分化为皮肤的各种细胞成分而有助于伤口修复[112],还能通过分泌血管内皮生长因子、基质细胞衍生因子1和血管生成素1等促进内皮细胞的存活和增殖,从而促进创面血管形成[113]。创面血管生成在创面愈合和组织再生中起着关键作用,是组织修复(如氧气和营养)赖以生长的物质基础。血管新生包括内皮细胞和血管平滑肌细胞的增殖、迁移、分化,增强血流量和毛细血管网的形成[114]。 此过程中涉及了多种细胞因子,其中血管内皮生长因子能够促进血管内皮细胞的有丝分裂同时增加血管的通透性,进而在血管再生中发挥着极其重要的作用[115]。KLEINHEINZ等[116]研究显示由间充质干细胞分泌的生长因子如血管内皮生长因子和白细胞介素8,对血管前体的激活有着重要的作用。胡克苏等[117]也证明了大鼠烧伤后移植的间充质干细胞能通过抑制肿瘤坏死因子及白细胞介素1 的表达,减轻烧伤创面局部炎症反应,同时增加碱性成纤维细胞生长因子、血管内皮生长因子的表达量,促进创面血管的再生,进而促进创面的快速修复。AN等[118]研究证明间充质干细胞在皮肤创面愈合中可以通过调控细胞自噬进而促进创面内皮细胞血管生成。此外,当自噬抑制剂3-甲基腺嘌呤抑制间充质干细胞自噬时,抗氧化剂途径Keap1/Nrf2也被阻断,说明骨髓间充质干细胞通过自噬作用介导对氧化应激损伤细胞的保护作用[119],提示间充质干细胞通过调控自噬效应增强了其分泌血管内皮生长因子的能力,进一步促进了创面内皮细胞的血管生成。 另外有研究证明间充质干细胞可以通过调节体内外的自噬效应来减轻慢性高糖诱导的细胞损伤[120]。HAN等[121]研究进一步说明脐带间充质干细胞可以通过诱导自噬从而促进糖尿病的伤口愈合。以上研究表明,增强间充质干细胞的自噬活性同样有利于慢性创面组织细胞的再生和修复。这些研究均提示间充质干细胞移植创面后,可以通过调控自身的自噬效应与创面环境互为影响,协调统一,共同促进创面组织的愈合。"

| [1] GREENHALGH DG. Sepsis in the burn patient: a different problem than sepsis in the general population. Burns Trauma. 2017;5:23. [2] YUHUA S, LIGEN L, JIAKE C, et al. Effect of Poloxamer 188 on deepening of deep second-degree burn wounds in the early stage. Burns. 2012;38(1):95-101. [3] BAKSH D, YAO R, TUAN RS. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells. 2007;25(6):1384-1392. [4] TROYER DL, WEISS ML. Wharton’s jelly-derived cells are a primitive stromal cell population. Stem Cells. 2008;26(3):591-599. [5] HUA J, GONG J, MENG H, et al. Comparison of different methods for the isolation of mesenchymal stem cells from umbilical cord matrix: proliferation and multilineage differentiation as compared to mesenchymal stem cells from umbilical cord blood and bone marrow. Cell Biol Int. 2014;38(2):198-210. [6] OH EJ, LEE HW, KALIMUTHU S, et al. In vivo migration of mesenchymal stem cells to burn injury sites and their therapeutic effects in a living mouse model. J Control Release. 2018;279:79-88. [7] LIU L, YU Y, HOU Y, et al. Human umbilical cord mesenchymal stem cells transplantation promotes cutaneous wound healing of severe burned rats. PLoS One. 2014;9(2):e88348. [8] BARGUES L, PRAT M, LECLERC T, et al. Present and future of cell therapy in burns. Pathol Biol (Paris). 2011;59(3):e49-56. [9] YANG R, WANG J, CHEN X, et al. Epidermal Stem Cells in Wound Healing and Regeneration. Stem Cells Int. 2020;2020:9148310. [10] SHUPP JW, NASABZADEH TJ, ROSENTHAL DS, et al. A review of the local pathophysiologic bases of burn wound progression. J Burn Care Res. 2010; 31(6):849-873. [11] ZHANG D, CHEN Y, XU X, et al. Autophagy inhibits the mesenchymal stem cell aging induced by D-galactose through ROS/JNK/p38 signalling. Clin Exp Pharmacol Physiol. 2020;47(3):466-477. [12] LV B, LI F, HAN J, et al. Hif-1α Overexpression Improves Transplanted Bone Mesenchymal Stem Cells Survival in Rat MCAO Stroke Model. Front Mol Neurosci. 2017;10:80. [13] LV B, HUA T, LI F, et al. Hypoxia-inducible factor 1 α protects mesenchymal stem cells against oxygen-glucose deprivation-induced injury via autophagy induction and PI3K/AKT/mTOR signaling pathway. Am J Transl Res. 2017; 9(5):2492-2499. [14] BAIRD A, COSTANTINI T, COIMBRA R, et al. Injury, inflammation and the emergence of human-specific genes. Wound Repair Regen. 2016;24(3):602-606. [15] PULLAR JM, CARR AC, VISSERS MCM. The Roles of Vitamin C in Skin Health. Nutrients. 2017;9(8):866. [16] AOKI S, TODA S, ANDO T, et al. Bone marrow stromal cells, preadipocytes, and dermal fibroblasts promote epidermal regeneration in their distinctive fashions. Mol Biol Cell. 2004;15(10):4647-4657. [17] SÁNCHEZ L, GUTIERREZ-ARANDA I, LIGERO G, et al. Enrichment of human ESC-derived multipotent mesenchymal stem cells with immunosuppressive and anti-inflammatory properties capable to protect against experimental inflammatory bowel disease. Stem Cells. 2011;29(2):251-262. [18] FIORINA P, JUREWICZ M, AUGELLO A, et al. Immunomodulatory function of bone marrow-derived mesenchymal stem cells in experimental autoimmune type 1 diabetes. J Immunol. 2009;183(2):993-1004. [19] CENTENO C, MARKLE J, DODSON E, et al. Treatment of lumbar degenerative disc disease-associated radicular pain with culture-expanded autologous mesenchymal stem cells: a pilot study on safety and efficacy. J Transl Med. 2017;15(1):197. [20] BEN NASR M, VERGANI A, AVRUCH J, et al. Co-transplantation of autologous MSCs delays islet allograft rejection and generates a local immunoprivileged site. Acta Diabetol. 2015;52(5):917-927. [21] BRUNO S, DEREGIBUS MC, CAMUSSI G. The secretome of mesenchymal stromal cells: Role of extracellular vesicles in immunomodulation. Immunol Lett. 2015;168(2):154-158. [22] LI N, HUA J. Interactions between mesenchymal stem cells and the immune system. Cell Mol Life Sci. 2017;74(13):2345-2360. [23] MONTEMURRO T, VIGANÒ M, RAGNI E, et al. Angiogenic and anti-inflammatory properties of mesenchymal stem cells from cord blood: soluble factors and extracellular vesicles for cell regeneration. Eur J Cell Biol. 2016;95(6-7):228-238. [24] LI Z, WANG J, GAO F, et al. Human Adipose-Derived Stem Cells Delay Retinal Degeneration in Royal College of Surgeons Rats Through Anti-Apoptotic and VEGF-Mediated Neuroprotective Effects. Curr Mol Med. 2016;16(6):553-566. [25] OHKOUCHI S, BLOCK GJ, KATSHA AM, et al. Mesenchymal stromal cells protect cancer cells from ROS-induced apoptosis and enhance the Warburg effect by secreting STC1. Mol Ther. 2012;20(2):417-423. [26] LI L, ZHANG S, ZHANG Y, et al. Paracrine action mediate the antifibrotic effect of transplanted mesenchymal stem cells in a rat model of global heart failure. Mol Biol Rep. 2009;36(4):725-731. [27] CHEN L, TREDGET EE, WU PY, et al. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008;3(4):e1886. [28] SMITH AN, WILLIS E, CHAN VT, et al. Mesenchymal stem cells induce dermal fibroblast responses to injury. Exp Cell Res. 2010;316(1):48-54. [29] WU Y, CHEN L, SCOTT PG, et al. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25(10): 2648-2659. [30] ZHANG B, WANG M, GONG A, et al. HucMSC-Exosome Mediated-Wnt4 Signaling Is Required for Cutaneous Wound Healing. Stem Cells. 2015;33(7): 2158-2168. [31] YOSHIKAWA T, MITSUNO H, NONAKA I, et al. Wound therapy by marrow mesenchymal cell transplantation. Plast Reconstr Surg. 2008;121(3):860-877. [32] WALTER MN, WRIGHT KT, FULLER HR, et al. Mesenchymal stem cell-conditioned medium accelerates skin wound healing: an in vitro study of fibroblast and keratinocyte scratch assays. Exp Cell Res. 2010;316(7):1271-1281. [33] HU YX, HAN XS, JING Q. Autophagy in Development and Differentiation. Adv Exp Med Biol. 2019;1206:469-487. [34] LIU XC, LU JJ, CHEN YM, et al. Roles of autophagy onself-renewal and differentiation of mesenchymal stem cells. Hua Xi Kou Qiang Yi Xue Za Zhi. 2020;38(6):704-707. [35] MA Y, QI M, AN Y, et al. Autophagy controls mesenchymal stem cell properties and senescence during bone aging. Aging Cell. 2018;17(1):e12709. [36] ZHENG Y, HU CJ, ZHUO RH, et al. Inhibition of autophagy alleviates the senescent state of rat mesenchymal stem cells during long-term culture. Mol Med Rep. 2014;10(6):3003-3008. [37] FERRO F, SPELAT R, SHAW G, et al. Survival/Adaptation of Bone Marrow-Derived Mesenchymal Stem Cells After Long-Term Starvation Through Selective Processes. Stem Cells. 2019;37(6):813-827. [38] JUNG J, CHOI JH, LEE Y, et al. Human placenta-derived mesenchymal stem cells promote hepatic regeneration in CCl4 -injured rat liver model via increased autophagic mechanism. Stem Cells. 2013;31(8):1584-1596. [39] HU Y, HUANG Y, YI Y, et al. Single-cell RNA sequencing highlights transcription activity of autophagy-related genes during hematopoietic stem cell formation in mouse embryos. Autophagy. 2017;13(4):770-771. [40] SONG BQ, CHI Y, LI X, et al. Inhibition of Notch Signaling Promotes the Adipogenic Differentiation of Mesenchymal Stem Cells Through Autophagy Activation and PTEN-PI3K/AKT/mTOR Pathway. Cell Physiol Biochem. 2015; 36(5):1991-2002. [41] SBRANA FV, CORTINI M, AVNET S, et al. The Role of Autophagy in the Maintenance of Stemness and Differentiation of Mesenchymal Stem Cells. Stem Cell Rev Rep. 2016;12(6):621-633. [42] GU Z, TAN W, JI J, et al. Rapamycin reverses the senescent phenotype and improves immunoregulation of mesenchymal stem cells from MRL/lpr mice and systemic lupus erythematosus patients through inhibition of the mTOR signaling pathway. Aging (Albany NY). 2016;8(5):1102-1114. [43] WANG L, ZHANG HY, GAO B, et al. Tetramethylpyrazine Protects Against Glucocorticoid-Induced Apoptosis by Promoting Autophagy in Mesenchymal Stem Cells and Improves Bone Mass in Glucocorticoid-Induced Osteoporosis Rats. Stem Cells Dev. 2017;26(6):419-430. [44] XIAO M, LI L, LI C, et al. Role of autophagy and apoptosis in wound tissue of deep second-degree burn in rats. Acad Emerg Med. 2014;21(4):383-391. [45] KIMURA A, ISHIDA Y, NOSAKA M, et al. Autophagy in skin wounds: a novel marker for vital reactions. Int J Legal Med. 2015;129(3):537-541. [46] GIBSON SB. Investigating the role of reactive oxygen species in regulating autophagy. Methods Enzymol. 2013;528:217-235. [47] WANG Y, NARTISS Y, STEIPE B, et al. ROS-induced mitochondrial depolarization initiates PARK2/PARKIN-dependent mitochondrial degradation by autophagy. Autophagy. 2012;8(10):1462-1476. [48] LI GH, LIN XL, ZHANG H, et al. Ox-Lp(a) transiently induces HUVEC autophagy via an ROS-dependent PAPR-1-LKB1-AMPK-mTOR pathway. Atherosclerosis. 2015;243(1):223-235. [49] DU J, TENG RJ, GUAN T, et al. Role of autophagy in angiogenesis in aortic endothelial cells. Am J Physiol Cell Physiol. 2012;302(2):C383-391. [50] LIN CW, JAN MS, KUO JH. Exploring MicroRNA Expression Profiles Related to the mTOR Signaling Pathway in Mouse Embryonic Fibroblast Cells Treated with Polyethylenimine. Mol Pharm. 2015;12(8):2858-2868. [51] XIAO M, LI L, HU Q, et al. Rapamycin reduces burn wound progression by enhancing autophagy in deep second-degree burn in rats. Wound Repair Regen. 2013;21(6):852-859. [52] XU J, MA Y, ZHU X, et al. Enhanced autophagy promotes the clearance of Pseudomonas aeruginosa in diabetic rats with wounds. Ann Transl Med. 2020;8(21):1362. [53] DAI J, SUN Y, CHEN D, et al. Negative regulation of PI3K/AKT/mTOR axis regulates fibroblast proliferation, apoptosis and autophagy play a vital role in triptolide-induced epidural fibrosis reduction. Eur J Pharmacol. 2019; 864:172724. [54] ZHANG J, ZHANG C, JIANG X, et al. Involvement of autophagy in hypoxia-BNIP3 signaling to promote epidermal keratinocyte migration. Cell Death Dis. 2019;10(3):234. [55] LEVINE B, KROEMER G. Autophagy in the pathogenesis of disease. Cell. 2008;132(1):27-42. [56] PACKER M. Autophagy-dependent and -independent modulation of oxidative and organellar stress in the diabetic heart by glucose-lowering drugs. Cardiovasc Diabetol. 2020;19(1):62. [57] PARK H, KANG JH, LEE S. Autophagy in Neurodegenerative Diseases: A Hunter for Aggregates. Int J Mol Sci. 2020;21(9):3369. [58] DE DUVE C, WATTIAUX R. Functions of lysosomes. Annu Rev Physiol. 1966; 28:435-492. [59] BOLENDER RP, WEIBEL ER. A morphometric study of the removal of phenobarbital-induced membranes from hepatocytes after cessation of threatment. J Cell Biol. 1973;56(3):746-761. [60] LEMASTERS JJ, NIEMINEN AL, QIAN T, et al. The mitochondrial permeability transition in cell death: a common mechanism in necrosis, apoptosis and autophagy. Biochim Biophys Acta. 1998;1366(1-2):177-196. [61] YIN H, WU H, CHEN Y, et al. The Therapeutic and Pathogenic Role of Autophagy in Autoimmune Diseases. Front Immunol. 2018;9:1512. [62] KLIONSKY DJ. Autophagy: from phenomenology to molecular understanding in less than a decade. Nat Rev Mol Cell Biol. 2007;8(11):931-937. [63] MAIURI MC, ZALCKVAR E, KIMCHI A, et al. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007; 8(9):741-752. [64] MIZUSHIMA N, KLIONSKY DJ. Protein turnover via autophagy: implications for metabolism. Annu Rev Nutr. 2007;27:19-40. [65] MIZUSHIMA N, KOMATSU M. Autophagy: renovation of cells and tissues. Cell. 2011;147(4):728-741. [66] CECCONI F, LEVINE B. The role of autophagy in mammalian development: cell makeover rather than cell death. Dev Cell. 2008;15(3):344-357. [67] ZHANG J, WANG P, WAN L, et al. The emergence of noncoding RNAs as Heracles in autophagy. Autophagy. 2017;13(6):1004-1024. [68] RASTALDO R, VITALE E, GIACHINO C. Dual Role of Autophagy in Regulation of Mesenchymal Stem Cell Senescence. Front Cell Dev Biol. 2020;8:276. [69] ZHANG H, ZHANG L, GAO B, et al. Golgi apparatus-localized synaptotagmin 2 is required for unconventional secretion in Arabidopsis. PLoS One. 2011; 6(11):e26477. [70] VAN HUIZEN E, MCINERNEY GM. Activation of the PI3K-AKT Pathway by Old World Alphaviruses. Cells. 2020;9(4):970. [71] LUND-RICARD Y, CORMIER P, MORALES J, et al. mTOR Signaling at the Crossroad between Metazoan Regeneration and Human Diseases. Int J Mol Sci. 2020;21(8):2718. [72] INOKI K, GUAN KL. Complexity of the TOR signaling network. Trends Cell Biol. 2006;16(4):206-212. [73] YURUBE T, ITO M, KAKIUCHI Y, et al. Autophagy and mTOR signaling during intervertebral disc aging and degeneration. JOR Spine. 2020;3(1):e1082. [74] ZHU Z, YANG C, IYASWAMY A, et al. Balancing mTOR Signaling and Autophagy in the Treatment of Parkinson’s Disease. Int J Mol Sci. 2019;20(3):728. [75] JUNG CH, JUN CB, RO SH, et al. ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol Biol Cell. 2009;20(7):1992-2003. [76] KALEAĞASIOĞLU F, ALI DM, BERGER MR. Multiple Facets of Autophagy and the Emerging Role of Alkylphosphocholines as Autophagy Modulators. Front Pharmacol. 2020;11:547. [77] KIM J, KUNDU M, VIOLLET B, et al. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13(2):132-141. [78] LU X, PALIOGIANNIS P, CALVISI DF, et al. Role of the Mammalian Target of Rapamycin Pathway in Liver Cancer: From Molecular Genetics to Targeted Therapies. Hepatology. 2021;73 Suppl 1(Suppl 1):49-61. [79] NOURIAN DEHKORDI A, MIRAHMADI BABAHEYDARI F, CHEHELGERDI M, et al. Skin tissue engineering: wound healing based on stem-cell-based therapeutic strategies. Stem Cell Res Ther. 2019;10(1):111. [80] LOZITO TP, TUAN RS. Mesenchymal stem cells inhibit both endogenous and exogenous MMPs via secreted TIMPs. J Cell Physiol. 2011;226(2):385-396. [81] JEON YK, JANG YH, YOO DR, et al. Mesenchymal stem cells’ interaction with skin: wound-healing effect on fibroblast cells and skin tissue. Wound Repair Regen. 2010;18(6):655-661. [82] LI C, LI G, LIU M, et al. Paracrine effect of inflammatory cytokine-activated bone marrow mesenchymal stem cells and its role in osteoblast function. J Biosci Bioeng. 2016;121(2):213-219. [83] ARNO AI, AMINI-NIK S, BLIT PH, et al. Human Wharton’s jelly mesenchymal stem cells promote skin wound healing through paracrine signaling. Stem Cell Res Ther. 2014 ;5(1):28. [84] WANG K, LIU R, LI J, et al. Quercetin induces protective autophagy in gastric cancer cells: involvement of Akt-mTOR- and hypoxia-induced factor 1α-mediated signaling. Autophagy. 2011;7(9):966-978. [85] RAMHORMOZI P, MOHAJER ANSARI J, SIMORGH S, et al. Bone Marrow-Derived Mesenchymal Stem Cells Combined With Simvastatin Accelerates Burn Wound Healing by Activation of the Akt/mTOR Pathway. J Burn Care Res. 2020;41(5):1069-1078. [86] WANG C, MAO C, LOU Y, et al. Monotropein promotes angiogenesis and inhibits oxidative stress-induced autophagy in endothelial progenitor cells to accelerate wound healing. J Cell Mol Med. 2018;22(3):1583-1600. [87] 汪涌,何清濂,林子豪.前列腺素E2(PGE2)对瘢痕成纤维细胞Ⅰ、Ⅲ型前胶原基因表达的影响[J].实用美容整形外科杂志,2001,12(1):35-37. [88] LAWRENCE J, NHO R. The Role of the Mammalian Target of Rapamycin (mTOR) in Pulmonary Fibrosis. Int J Mol Sci. 2018;19(3):778. [89] TEE AR. The Target of Rapamycin and Mechanisms of Cell Growth. Int J Mol Sci. 2018;19(3):880. [90] XU Y, TAI W, QU X, et al. Rapamycin protects against paraquat-induced pulmonary fibrosis: Activation of Nrf2 signaling pathway. Biochem Biophys Res Commun. 2017;490(2):535-540. [91] YOON JY, PARK CG, PARK BS, et al. Effects of Remifentanil Preconditioning Attenuating Oxidative Stress in Human Dermal Fibroblast. Tissue Eng Regen Med. 2017;14(2):133-141. [92] LI L, ZHANG J, ZHANG Q, et al. High Glucose Suppresses Keratinocyte Migration Through the Inhibition of p38 MAPK/Autophagy Pathway. Front Physiol. 2019;10:24. [93] LI XP, LIU P, LI YF, et al. LPS induces activation of the TLR4 pathway in fibroblasts and promotes skin scar formation through collagen I and TGF-β in skin lesions. Int J Clin Exp Pathol. 2019;12(6):2121-2129. [94] 王玉路,薛现军,刘敏洁,等.纤维细胞生长因子1对转化生长因子β1诱导的人肾成纤维细胞活化纤维化的保护作用[J].临床与病理杂志, 2019,39(12):2646-2652. [95] GONÇALVES JUNIOR R, PINHEIRO ADA R, SCHOICHET JJ, et al. MMP13, TIMP2 and TGFB3 Gene Polymorphisms in Brazilian Chronic Periodontitis and Periimplantitis Subjects. Braz Dent J. 2016;27(2):128-134. [96] LIU X, GU X, SUN L, et al. Downregulation of Smurf2, a tumor-suppressive ubiquitin ligase, in triple-negative breast cancers: involvement of the RB-microRNA axis. BMC Cancer. 2014;14:57. [97] KIM SI, NA HJ, DING Y, et al. Autophagy promotes intracellular degradation of type I collagen induced by transforming growth factor (TGF)-β1. J Biol Chem. 2012;287(15):11677-11688. [98] SUZUKI HI, KIYONO K, MIYAZONO K. Regulation of autophagy by transforming growth factor-β (TGF-β) signaling. Autophagy. 2010;6(5):645-647. [99] YU Y, YANG FH, ZHANG WT, et al. Mesenchymal stem cells desensitize castration-resistant prostate cancer to docetaxel chemotherapy via inducing TGF-β1-mediated cell autophagy. Cell Biosci. 2021;11(1):7. [100] SUN J, ZHOU Y, YE Z, et al. Transforming growth factor-β1 stimulates mesenchymal stem cell proliferation by altering cell cycle through FAK-Akt-mTOR pathway. Connect Tissue Res. 2019;60(4):406-417. [101] KOSARIC N, KIWANUKA H, GURTNER GC. Stem cell therapies for wound healing. Expert Opin Biol Ther. 2019;19(6):575-585. [102] SHOHARA R, YAMAMOTO A, TAKIKAWA S, et al. Mesenchymal stromal cells of human umbilical cord Wharton’s jelly accelerate wound healing by paracrine mechanisms. Cytotherapy. 2012;14(10):1171-1181. [103] KIM JW, LEE JH, LYOO YS, et al. The effects of topical mesenchymal stem cell transplantation in canine experimental cutaneous wounds. Vet Dermatol. 2013;24(2):242-e53. [104] 史永平,原博.脂肪干细胞对转化生长因子β1干预下真皮成纤维细胞致纤维化能力的同步影响[J].上海交通大学学报(医学版),2019, 39(12):1382-1388. [105] TAKAHAMA M, AKIRA S, SAITOH T. Autophagy limits activation of the inflammasomes. Immunol Rev. 2018;281(1):62-73. [106] MANFERDINI C, MAUMUS M, GABUSI E, et al. Adipose-derived mesenchymal stem cells exert antiinflammatory effects on chondrocytes and synoviocytes from osteoarthritis patients through prostaglandin E2. Arthritis Rheum. 2013;65(5):1271-1281. [107] ANDREOLI A, RUF MT, ITIN P, et al. Phosphorylation of the ribosomal protein S6, a marker of mTOR (mammalian target of rapamycin) pathway activation, is strongly increased in hypertrophic scars and keloids. Br J Dermatol. 2015; 172(5):1415-1417. [108] XIE J, LIU B, CHEN J, et al. Umbilical cord-derived mesenchymal stem cells alleviated inflammation and inhibited apoptosis in interstitial cystitis via AKT/mTOR signaling pathway. Biochem Biophys Res Commun. 2018;495(1): 546-552. [109] GAN L, SHEN H, LI X, et al. Mesenchymal stem cells promote chemoresistance by activating autophagy in intrahepatic cholangiocarcinoma. Oncol Rep. 2021;45(1):107-118. [110] TIAN J, KOU X, WANG R, et al. Autophagy controls mesenchymal stem cell therapy in psychological stress colitis mice. Autophagy. 2020:1-18. [111] LI R, TIAN J, DU J, et al. Manipulation of autophagy: a novelly potential therapeutic strategy for retinal neovascularization. BMC Ophthalmol. 2018; 18(1):110. [112] SASAKI M, ABE R, FUJITA Y, et al. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol. 2008;180(4):2581-2587. [113] LI M, ZHAO Y, HAO H, et al. Mesenchymal stem cell-based therapy for nonhealing wounds: today and tomorrow. Wound Repair Regen. 2015;23(4): 465-482. [114] BAZIGOU E, MAKINEN T. Flow control in our vessels: vascular valves make sure there is no way back. Cell Mol Life Sci. 2013;70(6):1055-1066. [115] DAI J, RABIE AB. VEGF: an essential mediator of both angiogenesis and endochondral ossification. J Dent Res. 2007;86(10):937-950. [116] KLEINHEINZ J, WIESMANN HP, STRATMANN U, et al. Evaluating angiogenesis and osteogenesis modified by vascular endothelial growth factor (VEGF). Mund Kiefer Gesichtschir. 2002;6(3):175-182. [117] 胡克苏,祁俊,蔡玉辉,等.人脐带间充质干细胞移植修复烧伤皮肤的机制[J].江苏医药,2014,40(7):759-761. [118] AN Y, LIU WJ, XUE P, et al. Autophagy promotes MSC-mediated vascularization in cutaneous wound healing via regulation of VEGF secretion. Cell Death Dis. 2018;9(2):58. [119] ZHANG Q, CHENG X, ZHANG H, et al. Dissecting molecular mechanisms underlying H2O2-induced apoptosis of mouse bone marrow mesenchymal stem cell: role of Mst1 inhibition. Stem Cell Res Ther. 2020;11(1):526. [120] ZHAO K, HAO H, LIU J, et al. Bone marrow-derived mesenchymal stem cells ameliorate chronic high glucose-induced β-cell injury through modulation of autophagy. Cell Death Dis. 2015;6(9):e1885. [121] HAN YF, SUN TJ, HAN YQ, et al. Clinical perspectives on mesenchymal stem cells promoting wound healing in diabetes mellitus patients by inducing autophagy. Eur Rev Med Pharmacol Sci. 2015;19(14):2666-2670. |
| [1] | Zhu Chan, Han Xuke, Yao Chengjiao, Zhou Qian, Zhang Qiang, Chen Qiu. Human salivary components and osteoporosis/osteopenia [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1439-1444. |
| [2] | Jin Tao, Liu Lin, Zhu Xiaoyan, Shi Yucong, Niu Jianxiong, Zhang Tongtong, Wu Shujin, Yang Qingshan. Osteoarthritis and mitochondrial abnormalities [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1452-1458. |
| [3] | Zhang Lichuang, Xu Hao, Ma Yinghui, Xiong Mengting, Han Haihui, Bao Jiamin, Zhai Weitao, Liang Qianqian. Mechanism and prospects of regulating lymphatic reflux function in the treatment of rheumatoid arthritis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1459-1466. |
| [4] | Li Qin, Mao Shuangfa, Li Min, Cheng Jiyan. Protective effect and mechanism of dendrobium on fibroblasts damaged by ultraviolet B [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1228-1233. |
| [5] | Wang Jing, Xiong Shan, Cao Jin, Feng Linwei, Wang Xin. Role and mechanism of interleukin-3 in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1260-1265. |
| [6] | Xiao Hao, Liu Jing, Zhou Jun. Research progress of pulsed electromagnetic field in the treatment of postmenopausal osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1266-1271. |
| [7] | Zhu Chan, Han Xuke, Yao Chengjiao, Zhang Qiang, Liu Jing, Shao Ming. Acupuncture for Parkinson’s disease: an insight into the action mechanism in animal experiments [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1272-1277. |
| [8] | Wang Xinmin, Liu Fei, Xu Jie, Bai Yuxi, Lü Jian. Core decompression combined with dental pulp stem cells in the treatment of steroid-associated femoral head necrosis in rabbits [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1074-1079. |
| [9] | Fang Xiaolei, Leng Jun, Zhang Chen, Liu Huimin, Guo Wen. Systematic evaluation of different therapeutic effects of mesenchymal stem cell transplantation in the treatment of ischemic stroke [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1085-1092. |
| [10] | Guo Jia, Ding Qionghua, Liu Ze, Lü Siyi, Zhou Quancheng, Gao Yuhua, Bai Chunyu. Biological characteristics and immunoregulation of exosomes derived from mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1093-1101. |
| [11] | Wu Weiyue, Guo Xiaodong, Bao Chongyun. Application of engineered exosomes in bone repair and regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1102-1106. |
| [12] | Zhou Hongqin, Wu Dandan, Yang Kun, Liu Qi. Exosomes that deliver specific miRNAs can regulate osteogenesis and promote angiogenesis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1107-1112. |
| [13] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [14] | Huang Chenwei, Fei Yankang, Zhu Mengmei, Li Penghao, Yu Bing. Important role of glutathione in stemness and regulation of stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1119-1124. |
| [15] | Hui Xiaoshan, Bai Jing, Zhou Siyuan, Wang Jie, Zhang Jinsheng, He Qingyong, Meng Peipei. Theoretical mechanism of traditional Chinese medicine theory on stem cell induced differentiation [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1125-1129. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||