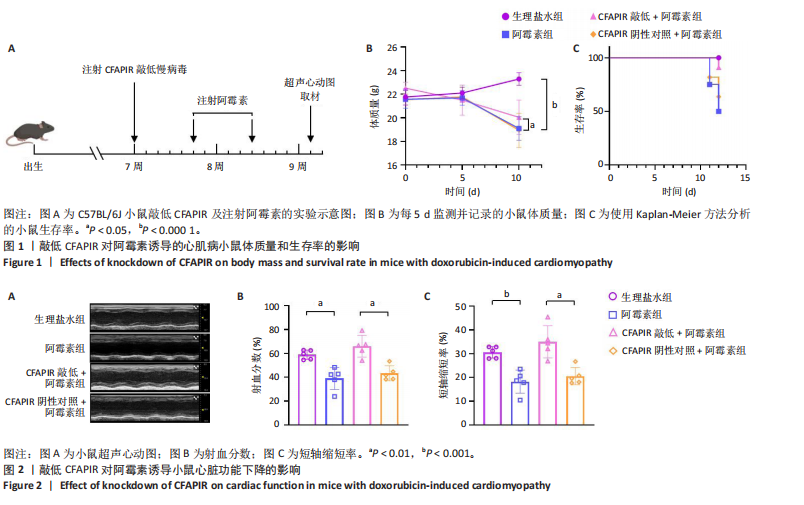
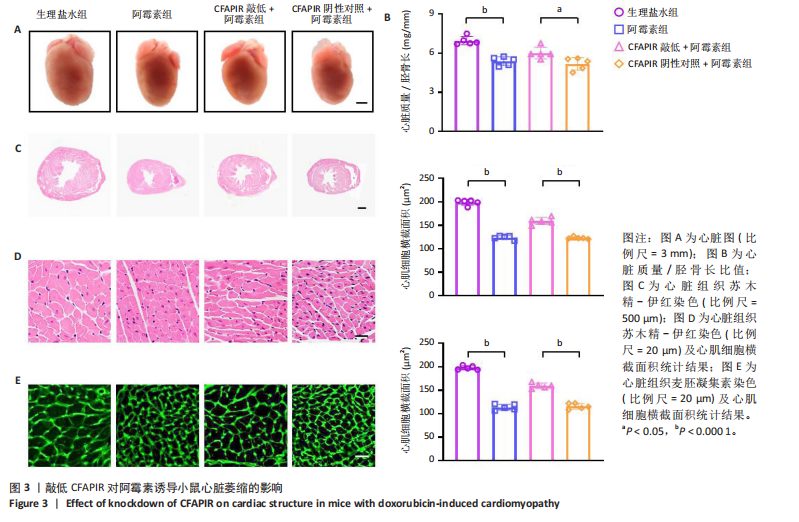
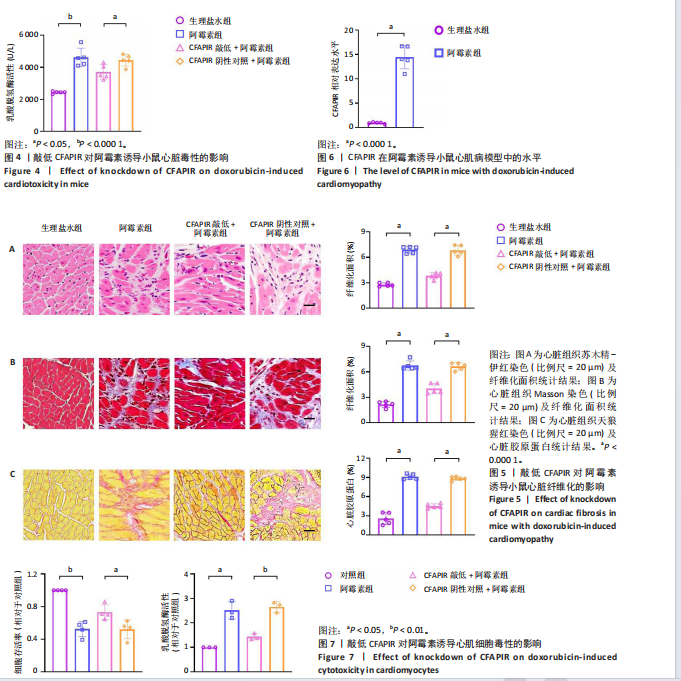
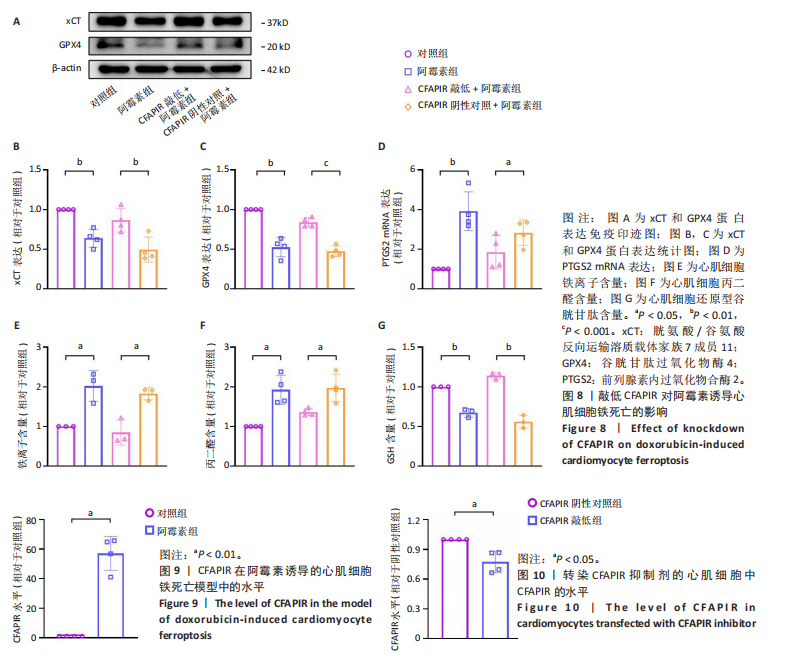
[1] CHEN Y, SHI S, DAI Y. Research progress of therapeutic drugs for doxorubicin-induced cardiomyopathy. Biomed Pharmacother. 2022; 156:113903.
[2] WILLIAMS PA, ZAIDI SK, SENGUPTA R. AACR Cancer Progress Report 2023: Advancing the Frontiers of Cancer Science and Medicine. Clin Cancer Res. 2023;29(19):3850-3851.
[3] LI D, YANG Y, WANG S, et al. Role of acetylation in doxorubicin-induced cardiotoxicity. Redox Biol. 2021;46:102089.
[4] CHRISTIDI E, BRUNHAM LR. Regulated cell death pathways in doxorubicin-induced cardiotoxicity. Cell Death Dis. 2021;12(4):339.
[5] WU L, ZHANG Y, WANG G, et al. Molecular Mechanisms and Therapeutic Targeting of Ferroptosis in Doxorubicin-Induced Cardiotoxicity. JACC Basic Transl Sci. 2024;9(6):811-826.
[6] VITALE R, MARZOCCO S, POPOLO A. Role of Oxidative Stress and Inflammation in Doxorubicin-Induced Cardiotoxicity: A Brief Account. Int J Mol Sci. Int J Mol Sci. 2024;25(13):7477.
[7] FABIANI I, AIMO A, GRIGORATOS C, et al. Oxidative stress and inflammation: determinants of anthracycline cardiotoxicity and possible therapeutic targets. Heart Fail Rev. 2021;26(4):881-890.
[8] SCHIRONE L, D’AMBROSIO L, FORTE M, et al.Mitochondria and Doxorubicin-Induced Cardiomyopathy: A Complex Interplay. Cells. 2022;11(13):2000.
[9] BELGER C, ABRAHAMS C, IMAMDIN A, et al. Doxorubicin-induced cardiotoxicity and risk factors. Int J Cardiol Heart Vasc. 2024;50:101332.
[10] FANG X, ARDEHALI H, MIN J, et al. The molecular and metabolic landscape of iron and ferroptosis in cardiovascular disease. Nat Rev Cardiol. 2023;20(1):7-23.
[11] DIXON SJ, LEMBERG KM, LAMPRECHT MR, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death]. Cell. 2012;149(5):1060-1072.
[12] WU YT, ZHANG GY, LI L, et al. Salvia miltiorrhiza suppresses cardiomyocyte ferroptosis after myocardial infarction by activating Nrf2 signaling. J Ethnopharmacol. 2024;330:118214.
[13] QU Z, PANG X, MEI Z, et al. The positive feedback loop of the NAT10/Mybbp1a/p53 axis promotes cardiomyocyte ferroptosis to exacerbate cardiac I/R injury. Redox Biol. 2024;72:103145.
[14] CUI J, CHEN Y, YANG Q, et al. Protosappanin A Protects DOX-Induced Myocardial Injury and Cardiac Dysfunction by Targeting ACSL4/FTH1 Axis-Dependent Ferroptosis. Adv Sci (Weinh). 2024;11(34): e2310227.
[15] LIU P, ZHANG Z, CAI Y, et al. Ferroptosis: Mechanisms and role in diabetes mellitus and its complications. Ageing Res Rev. 2024;94: 102201.
[16] ZHANG W, QIAN S, TANG B, et al. Resveratrol inhibits ferroptosis and decelerates heart failure progression via Sirt1/p53 pathway activation. J Cell Mol Med. 2023;27(20):3075-3089.
[17] GONG Y, YANG H, CHEN T, et al. USP38 exacerbates myocardial injury and malignant ventricular arrhythmias after ischemia/reperfusion by promoting ferroptosis through the P53/SLC7A11 pathway. Int Immunopharmacol. 2025;145:113727.
[18] XU X, XU XD, MA MQ, et al. The mechanisms of ferroptosis and its role in atherosclerosis. Biomed Pharmacother. 2024;171:116112.
[19] HE X, XIONG Y, LIU Y, et al. Ferrostatin-1 inhibits ferroptosis of vascular smooth muscle cells and alleviates abdominal aortic aneurysm formation through activating the SLC7A11/GPX4 axis. Faseb J. 2024; 38(2):e23401.
[20] QIU H, HUANG S, LIU Y, et al. Idebenone alleviates doxorubicin-induced cardiotoxicity by stabilizing FSP1 to inhibit ferroptosis. Acta Pharm Sin B. 2024;14(6):2581-2597.
[21] YANG Y, REN J, ZHANG J, et al. FTO ameliorates doxorubicin-induced cardiotoxicity by inhibiting ferroptosis via P53-P21/Nrf2 activation in a HuR-dependent m6A manner. Redox Biol. 2024;70:103067.
[22] LIU D, CHENG X, WU H, et al. CREG1 attenuates doxorubicin-induced cardiotoxicity by inhibiting the ferroptosis of cardiomyocytes. Redox Biol. 2024;75:103293.
[23] WU L, DU Y, WANG L, et al. Inhibition of METTL3 ameliorates doxorubicin-induced cardiotoxicity through suppression of TFRC-mediated ferroptosis. Redox Biol. 2024;72:103157.
[24] FANG X, WANG H, HAN D, et al. Ferroptosis as a target for protection against cardiomyopathy. Proc Natl Acad Sci U S A. 2019;116(7): 2672-2680.
[25] WANG K, LI FH, ZHOU LY, et al. HNEAP Regulates Necroptosis of Cardiomyocytes by Suppressing the m(5) C Methylation of Atf7 mRNA. Adv Sci (Weinh). 2023;10(34):e2304329.
[26] CHEN B, SHI B, ZHOU Z, et al. Targeting a cardiac abundant and fibroblasts-specific piRNA (CFRPi) to attenuate and reverse cardiac fibrosis in pressure-overloaded heart failure. Transl Res. 2024;267: 10-24.
[27] ZHOU Y, FANG Y, DAI C, et al. PiRNA pathway in the cardiovascular system: a novel regulator of cardiac differentiation, repair and regeneration. J Mol Med (Berl). 2021;99(12):1681-1690.
[28] WU Z, YU X, ZHANG S, et al. Novel roles of PIWI proteins and PIWI-interacting RNAs in human health and diseases. Cell Commun Signal. 2023;21(1):343.
[29] ZENG Q, WAN H, ZHAO S, et al. Role of PIWI-interacting RNAs on cell survival: Proliferation, apoptosis, and cycle. IUBMB Life. 2020;72(9): 1870-1878.
[30] GARCIA-BORJA E, SIEGL F, MATEU R, et al. Critical appraisal of the piRNA-PIWI axis in cancer and cancer stem cells. Biomark Res. 2024; 12(1):15.
[31] DENG X, LIAO T, XIE J, et al. The burgeoning importance of PIWI-interacting RNAs in cancer progression. Sci China Life Sci. 2024;67(4): 653-662.
[32] JIANG M, HONG X, GAO Y, et al. piRNA associates with immune diseases. Cell Commun Signal. 2024;22(1):347.
[33] PIEROULI K, PAPAKONSTANTINOU E, PAPAGEORGIOU L, et al. Role of non‑coding RNAs as biomarkers and the application of omics technologies in Alzheimer’s disease (Review). Int J Mol Med. 2023; 51(1):5.
[34] LI M, YANG Y, WANG Z, et al. Piwi-interacting RNAs (piRNAs) as potential biomarkers and therapeutic targets for cardiovascular diseases. Angiogenesis. 2021;24(1):19-34.
[35] LV L, YUAN K, LI J, et al. PiRNA CFAPIR inhibits cardiac fibrosis by regulating the muscleblind-like protein MBNL2. Biochim Biophys Acta Mol Basis Dis. 2024;1870(8):167456.
[36] GAO XQ, ZHANG YH, LIU F, et al. The piRNA CHAPIR regulates cardiac hypertrophy by controlling METTL3-dependent N(6)-methyladenosine methylation of Parp10 mRNA. Nat Cell Biol. 2020;22(11):1319-1331.
[37] WANG K, ZHOU LY, LIU F, et al. PIWI-Interacting RNA HAAPIR Regulates Cardiomyocyte Death After Myocardial Infarction by Promoting NAT10-Mediated ac(4) C Acetylation of Tfec mRNA. Adv Sci (Weinh). 2022;9(8):e2106058.
[38] CHI H, CHAI Y, MA L, et al. The mechanism by which piR-000699 targets SLC39A14 regulates ferroptosis in aging myocardial ischemia/reperfusion injury. Acta Biochim Biophys Sin (Shanghai). 2024;56(9):1352-1364.
[39] JIAO A, LIU H, WANG H, et al. piR112710 attenuates diabetic cardiomyopathy through inhibiting Txnip/NLRP3-mediated pyroptosis in db/db mice. Cell Signal. 2024;122:111333.
[40] LIU Y, QI H, ZONG J, et al. Oral Piwi-Interacting RNA Delivery Mediated by Green Tea-Derived Exosome-Like Nanovesicles for the Treatment of Aortic Dissection. Adv Healthc Mater. 2024;13(30):e2401466.
[41] HU C, ZHANG X, WEI W, et al. Matrine attenuates oxidative stress and cardiomyocyte apoptosis in doxorubicin-induced cardiotoxicity via maintaining AMPKα/UCP2 pathway. Acta Pharm Sin B. 2019;9(4):690-701.
[42] SANGOMLA S, SAIFI MA, KHURANA A, et al. Nanoceria ameliorates doxorubicin induced cardiotoxicity: Possible mitigation via reduction of oxidative stress and inflammation. J Trace Elem Med Biol. 2018; 47:53-62.
[43] ZHOU L, LI R, LIU C, et al.Foxo3a inhibits mitochondrial fission and protects against doxorubicin-induced cardiotoxicity by suppressing MIEF2.Free Radic Biol Med. 2017;104:360-370.
[44] TAI P, CHEN X, JIA G, et al. WGX50 mitigates doxorubicin-induced cardiotoxicity through inhibition of mitochondrial ROS and ferroptosis. J Transl Med. 2023;21(1):823.
[45] MENON A V, KIM J.Iron Promotes Cardiac Doxorubicin Retention and Toxicity Through Downregulation of the Mitochondrial Exporter ABCB8. Front Pharmacol. 2022;13:817951.
[46] KITAKATA H, ENDO J, IKURA H, et al. Therapeutic Targets for DOX-Induced Cardiomyopathy: Role of Apoptosis vs. Ferroptosis.Int J Mol Sci. 2022;23(3):1414.
[47] HUANG C, GUO Y, LI T, et al.Pharmacological activation of GPX4 ameliorates doxorubicin-induced cardiomyopathy.Redox Biol. 2024; 70:103024.
[48] KCIUK M, GIELECIŃSKA A, MUJWAR S, et al. Doxorubicin-An Agent with Multiple Mechanisms of Anticancer Activity. Cells. 2023;12(4):659.
[49] TADOKORO T, IKEDA M, IDE T, et al. Mitochondria-dependent ferroptosis plays a pivotal role in doxorubicin cardiotoxicity. JCI Insight. 2020;5(9):e132747.
[50] WANG X, RAMAT A, SIMONELIG M, et al. Emerging roles and functional mechanisms of PIWI-interacting RNAs. Nat Rev Mol Cell Biol. 2023; 24(2):123-141.
[51] ICHIKAWA Y, GHANEFAR M, BAYEVA M, et al. Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J Clin Invest. 2014;124(2):617-630.
[52] LIU Z, ZHAO X. piRNAs as emerging biomarkers and physiological regulatory molecules in cardiovascular disease. Biochem Biophys Res Commun. 2024;711:149906.
|