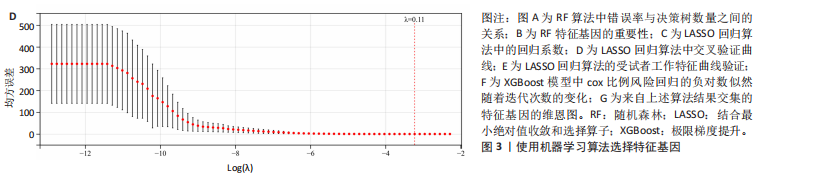
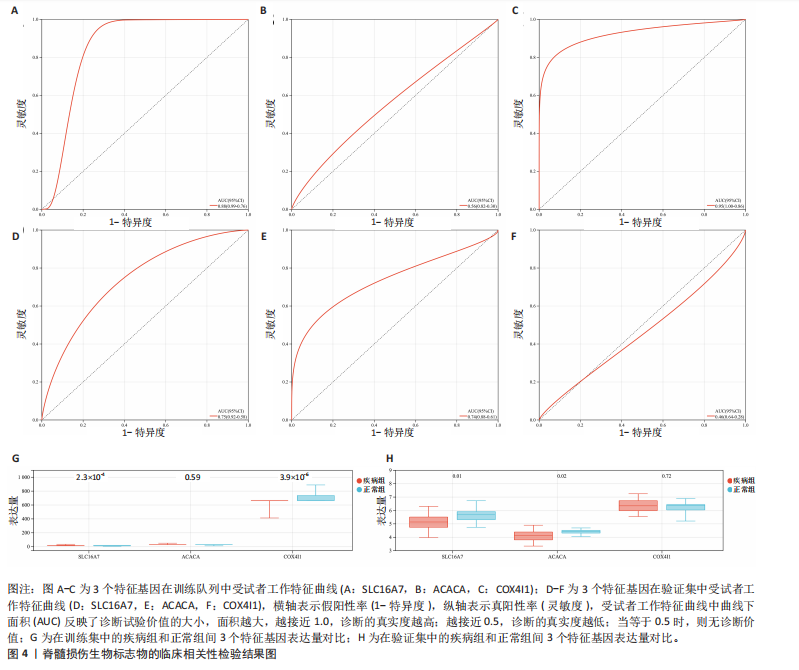
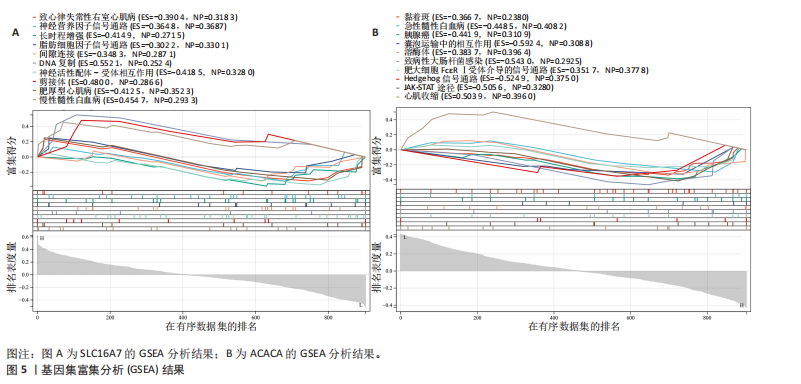
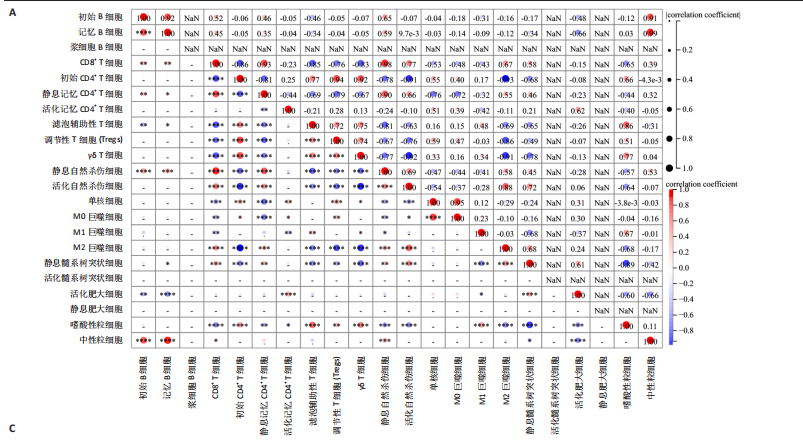
[1] MCDONALD JW, SADOWSKY C. Spinal-cord injury. Lancet. 2002;359(9304):417-425.
[2] ANJUM A, YAZID MD, FAUZI DAUD M, et al. Spinal Cord Injury: Pathophysiology, Multimolecular Interactions, and Underlying Recovery Mechanisms. Int J Mol Sci. 2020; 21(20):7533.
[3] HU X, XU W, REN Y, et al. Spinal cord injury: molecular mechanisms and therapeutic interventions. Signal Transduct Target Ther. 2023;8(1):245.
[4] GOLESTANI A, SHOBEIRI P, SADEGHI-NAINI M, et al. Epidemiology of Traumatic Spinal Cord Injury in Developing Countries from 2009 to 2020: A Systematic Review and Meta-Analysis. Neuroepidemiology. 2022;56(4):219-239.
[5] SHI Z, YUAN S, SHI L, et al. Programmed cell death in spinal cord injury pathogenesis and therapy. Cell Prolif. 2021;54(3):e12992.
[6] ZHENG B, TUSZYNSKI MH. Regulation of axonal regeneration after mammalian spinal cord injury. Nat Rev Mol Cell Biol. 2023;24(6):396-413.
[7] NAKAZAKI M, MORITA T, LANKFORD KL, et al. Small extracellular vesicles released by infused mesenchymal stromal cells target M2 macrophages and promote TGF-β upregulation, microvascular stabilization and functional recovery in a rodent model of severe spinal cord injury. J Extracell Vesicles. 2021;10(11):e12137.
[8] XIONG W, LI C, KONG G, et al. Treg cell-derived exosomes miR-709 attenuates microglia pyroptosis and promotes motor function recovery after spinal cord injury. J Nanobiotechnology. 2022;20(1):529.
[9] WANG Q, YI J, LIU H, et al. Iguratimod promotes functional recovery after SCI by repairing endothelial cell tight junctions. Exp Neurol. 2023;368:114503.
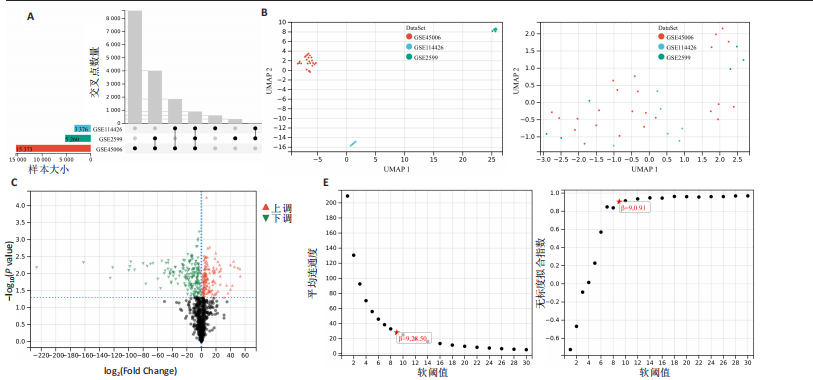
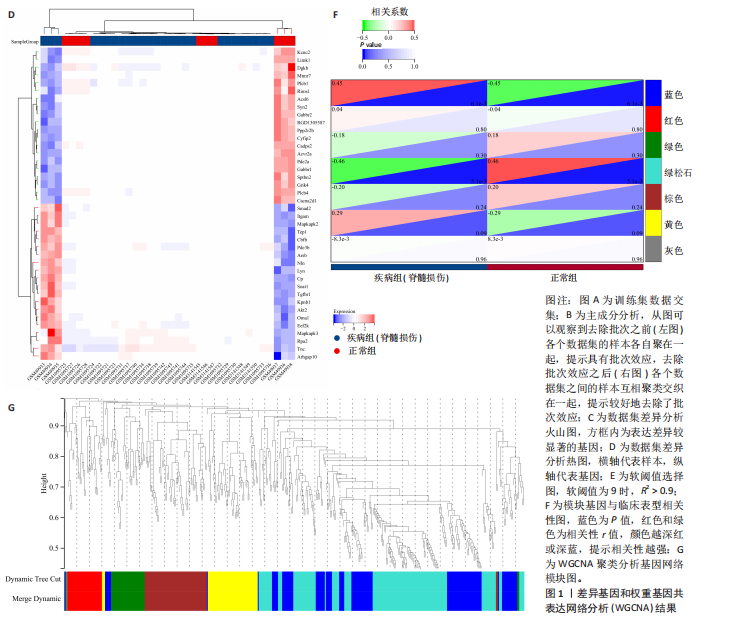
[10] WANG J, YANG P, YU T, et al. Lactylation of PKM2 Suppresses Inflammatory Metabolic Adaptation in Pro-inflammatory Macrophages. Int J Biol Sci. 2022;18(16): 6210-6225.
[11] ZHANG B, LI F, SHI Y, et al. Single-cell RNA sequencing integrated with bulk RNA sequencing analysis reveals the protective effects of lactate-mediated lactylation of microglia-related proteins on spinal cord injury. CNS Neurosci Ther. 2024;30(9): e70028.
[12] HU X, HUANG J, LI Z, et al. Lactate promotes microglial scar formation and facilitates locomotor function recovery by enhancing histone H4 lysine 12 lactylation after spinal cord injury. J Neuroinflammation. 2024;21(1):193.
[13] SANDERSON E, GLYMOUR MM, HOLMES MV, et al. Mendelian randomization. Nat Rev Methods Primers. 2022;2:6.
[14] CHANG X, ZHENG W, ZHAO Y, et al. Association of Lactate with Risk of Cardiovascular Diseases: A Two-Sample Mendelian Randomization Study. Vasc Health Risk Manag. 2024;20:541-551.
[15] RASOOLY D, PELOSO GM, PEREIRA AC, et al. Genome-wide association analysis and Mendelian randomization proteomics identify drug targets for heart failure. Nat Commun. 2023;14(1):3826.
[16] RAIES A, TULODZIECKA E, STAINER J, et al. DrugnomeAI is an ensemble machine-learning framework for predicting druggability of candidate drug targets. Commun Biol. 2022;5(1):1291.
[17] FANG S, DONG L, LIU L, et al. HERB: a high-throughput experiment- and reference-guided database of traditional Chinese medicine. Nucleic Acids Res. 2021;49(D1): D1197-D1206.
[18] TIAN S, ZHANG J, YUAN S, et al. Exploring pharmacological active ingredients of traditional Chinese medicine by pharmacotranscriptomic map in ITCM. Brief Bioinform. 2023;24(2):bbad027.
[19] HOEKSTRA SP, KAMIJO YI, MATSUSHITA T, et al. The acute effect of dopamine infusion on lipid and cytokine concentrations in persons with a cervical spinal cord injury-a pilot study. Spinal Cord. 2021;59(3):274-281.
[20] GAGLIANI N, AMEZCUA VESELY MC, ISEPPON A, et al. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature. 2015;523(7559): 221-225.
[21] ZHANG D, TANG Z, HUANG H, et al. Metabolic regulation of gene expression by histone lactylation. Nature. 2019; 574(7779):575-580.
[22] WANG X, FAN W, LI N, et al. YY1 lactylation in microglia promotes angiogenesis through transcription activation-mediated upregulation of FGF2. Genome Biol. 2023; 24(1):87.
[23] 葛玲玲,黄洪军,罗艳.乳酰化修饰在疾病中的作用及机制研究进展[J].上海交通大学学报(医学版),2023,43(3):374-379.
[24] CHEN H, LI Y, LI H, et al. NBS1 lactylation is required for efficient DNA repair and chemotherapy resistance. Nature. 2024;631(8021):663-669.
[25] ORIHUELA R, MCPHERSON CA, HARRY GJ. Microglial M1/M2 polarization and metabolic states. Br J Pharmacol. 2016; 173(4):649-665.
[26] JOSHI L, PLASTIRA I, BERNHART E, et al. Lysophosphatidic Acid Induces Aerobic Glycolysis, Lipogenesis, and Increased Amino Acid Uptake in BV-2 Microglia. Int J Mol Sci. 2021;22(4):1968.
[27] SUHAIL H, NEMATULLAH M, RASHID F, et al. An early glycolysis burst in microglia regulates mitochondrial dysfunction in oligodendrocytes under neuroinflammation. iScience. 2023;26(10):107921.
[28] DEVANNEY NA, STEWART AN, GENSEL JC. Microglia and macrophage metabolism in CNS injury and disease: The role of immunometabolism in neurodegeneration and neurotrauma. Exp Neurol. 2020;329: 113310.
[29] PROTO JD, DORAN AC, GUSAROVA G, et al. Regulatory T Cells Promote Macrophage Efferocytosis during Inflammation Resolution. Immunity. 2018;49(4):666-677.e6.
[30] PINHEIRO C, LONGATTO-FILHO A, SCAPULATEMPO C, et al. Increased expression of monocarboxylate transporters 1, 2, and 4 in colorectal carcinomas. Virchows Arch. 2008;452(2):139-146.
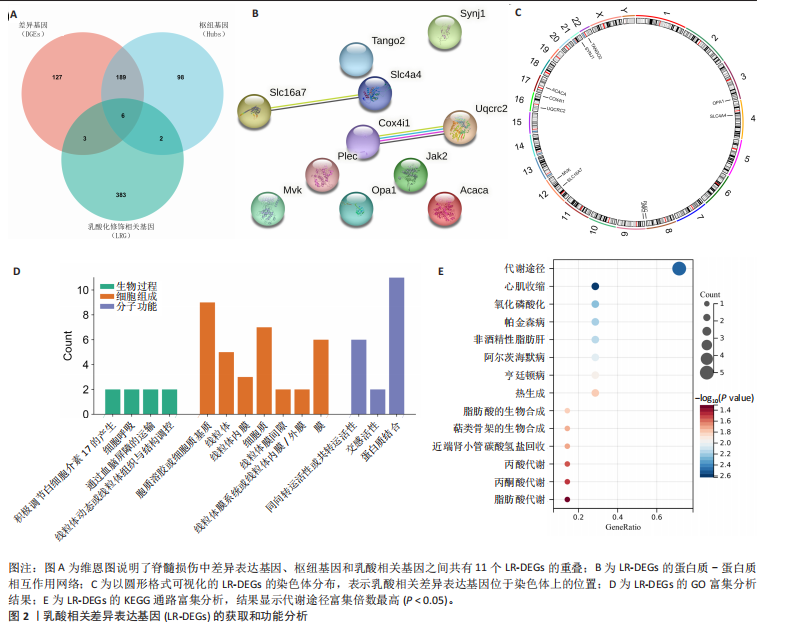
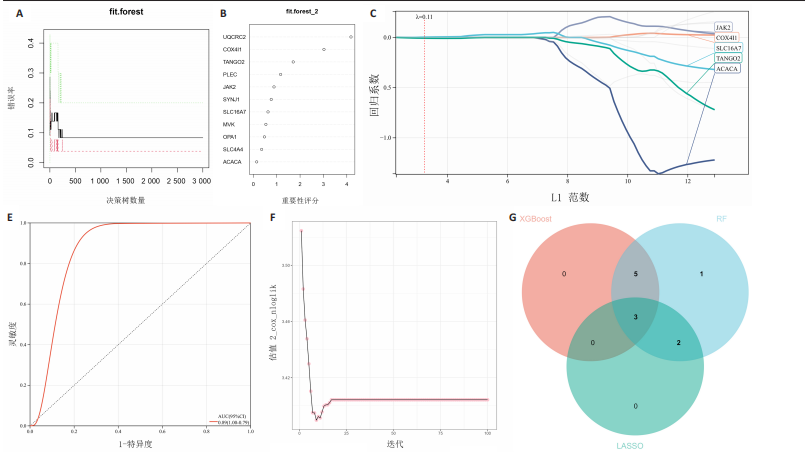
[31] PERTEGA-GOMES N, VIZCAINO JR, FELISBINO S, et al. Epigenetic and oncogenic regulation of SLC16A7 (MCT2) results in protein over-expression, impacting on signalling and cellular phenotypes in prostate cancer. Oncotarget. 2015;6(25):21675-21684.
[32] HALESTRAP AP. The SLC16 gene family - structure, role and regulation in health and disease. Mol Aspects Med. 2013;34(2-3): 337-349.
[33] LEE I, LEE SJ, KANG WK, et al. Inhibition of monocarboxylate transporter 2 induces senescence-associated mitochondrial dysfunction and suppresses progression of colorectal malignancies in vivo. Mol Cancer Ther. 2012;11(11):2342-2351.
[34] DONG J, LI M, PENG R, et al. ACACA reduces lipid accumulation through dual regulation of lipid metabolism and mitochondrial function via AMPK- PPARα- CPT1A axis. J Transl Med. 2024;22(1):196.
[35] GUO X, CHEN C, LIU B, et al. Genetic variations in monocarboxylate transporter genes as predictors of clinical outcomes in non-small cell lung cancer. Tumour Biol. 2015;36(5):3931-3939.
[36] CARMEL JB, GALANTE A, SOTEROPOULOS P, et al. Gene expression profiling of acute spinal cord injury reveals spreading inflammatory signals and neuron loss. Physiol Genomics. 2001;7(2):201-213.
[37] LI A, GONG Z, LONG Y, et al. Lactylation of LSD1 is an acquired epigenetic vulnerability of BRAFi/MEKi-resistant melanoma. Dev Cell. 2025. doi: 10.1016/j.devcel.2025.02.016.
[38] HU X, HUANG X, YANG Y, et al. Dux activates metabolism-lactylation-MET network during early iPSC reprogramming with Brg1 as the histone lactylation reader. Nucleic Acids Res. 2024;52(10):5529-5548.
[39] CHEN X, WANG J, CHAN P, et al. Metabolic Reprogramming in Spinal Cord Injury and Analysis of Potential Therapeutic Targets. J Mol Neurosci. 2025;75(2):50.
[40] 周逸敏,李宗洋,许翰勋,等.中药改善脊髓微环境修复血-脊髓屏障的机制研究进展[J].中国中医骨伤科杂志,2023, 31(9):80-83.
[41] 郭铁峰,江朔轩,张彦军,等.微血管内皮细胞在脊髓损伤中的作用机制及中药干预脊髓损伤的研究进展[J].中国脊柱脊髓杂志,2024,34(6):647-651.
[42] 钟远鸣,叶伟权,邱伟,等.中医药治疗脊髓损伤相关并发症的研究进展[J].海南医学院学报,2023,29(11):866-871.
[43] SQUAIR JW, MILANO M, DE COUCY A, et al. Recovery of walking after paralysis by regenerating characterized neurons to their natural target region. Science. 2023;381(6664):1338-1345.
[44] SENGELAUB DR, HAN Q, LIU NK, et al. Protective Effects of Estradiol and Dihydrotestosterone following Spinal Cord Injury. J Neurotrauma. 2018;35(6):825-841.
[45] MLCEK J, JURIKOVA T, SKROVANKOVA S, et al. Quercetin and Its Anti-Allergic Immune Response. Molecules. 2016;21(5):623.
[46] WANG X, FU Y, BOTCHWAY BOA, et al. Quercetin Can Improve Spinal Cord Injury by Regulating the mTOR Signaling Pathway. Front Neurol. 2022;13:905640.
[47] LIPINSKI CA, LOMBARDO F, DOMINY BW, et al. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46(1-3):3-26.
[48] 李盛华,柴喜平,王想福,等.中医药治疗脊髓损伤的研究进展[J].中国中医骨伤科杂志,2010,18(11):70-72.
[49] SNG KS, LI G, ZHOU LY, et al. Ginseng extract and ginsenosides improve neurological function and promote antioxidant effects in rats with spinal cord injury: A meta-analysis and systematic review. J Ginseng Res. 2022; 46(1):11-22.
[50] YUKSEL Y, GUVEN M, KAYMAZ B, et al. Effects of Aloe Vera on Spinal Cord Ischemia-Reperfusion Injury of Rats. J Invest Surg. 2016;29(6):389-398.
[51] GOKCE EC, KAHVECI R, ATANUR OM, et al. Neuroprotective effects of Ganoderma lucidum polysaccharides against traumatic spinal cord injury in rats. Injury. 2015; 46(11):2146-2155. |