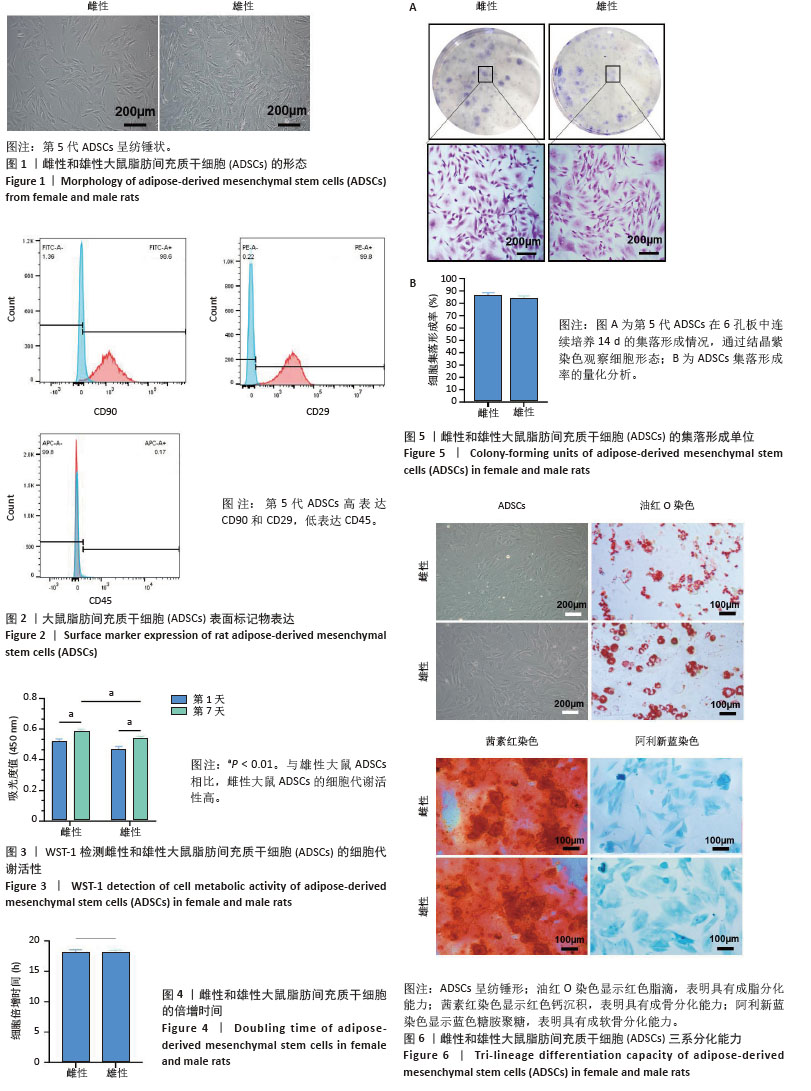
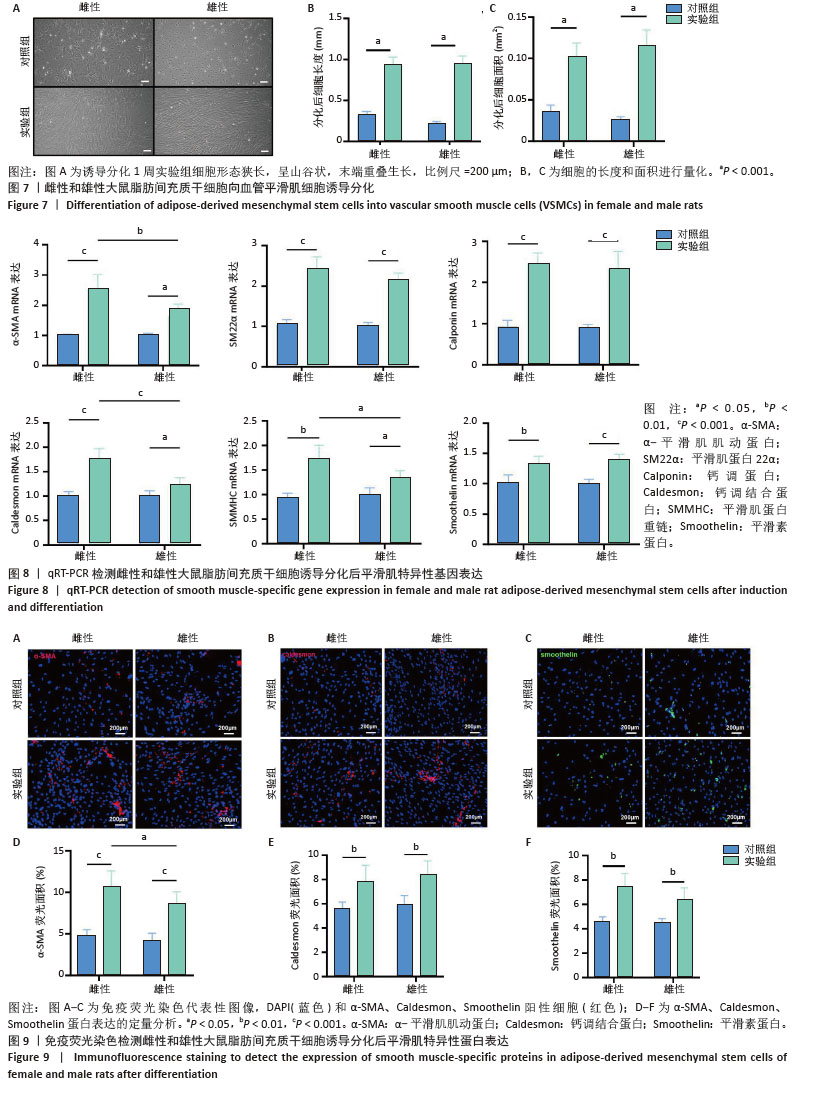
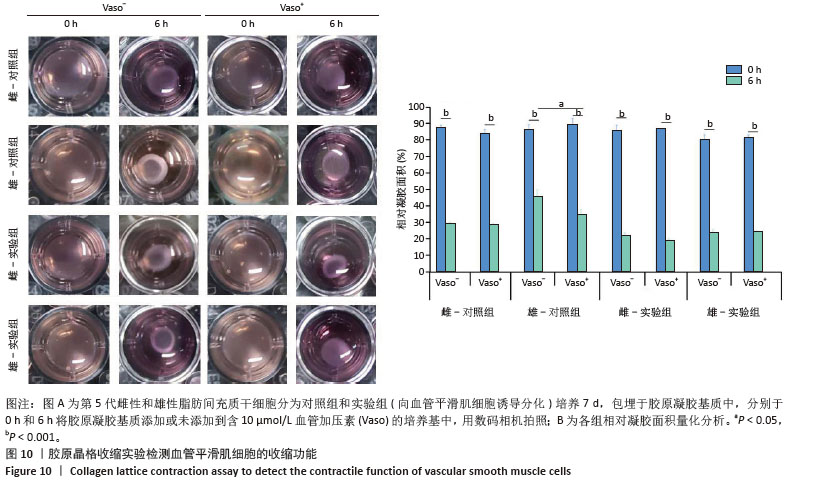
[1] MCCLELLAN M, BROWN N, CALIFF RM, et al. Call to Action: Urgent Challenges in Cardiovascular Disease: A Presidential Advisory From the American Heart Association. Circulation. 2019;139(9):e44-e54.
[2] SMAGUL S, KIM Y, SMAGULOVA A, et al. Biomaterials Loaded with Growth Factors/Cytokines and Stem Cells for Cardiac Tissue Regeneration. Int J Mol Sci. 2020;21(17):5952.
[3] MONGUIÓ-TORTAJADA M, PRAT-VIDAL C, MORON-FONT M, et al. Local administration of porcine immunomodulatory, chemotactic and angiogenic extracellular vesicles using engineered cardiac scaffolds for myocardial infarction. Bioact Mater. 2021;6(10):3314-3327.
[4] QIN K, WANG F, SIMPSON RML, et al. Hyaluronan promotes the regeneration of vascular smooth muscle with potent contractile function in rapidly biodegradable vascular grafts. Biomaterials. 2020; 257:120226.
[5] MI CH, QI XY, ZHOU YW, et al. Advances in medical polyesters for vascular tissue engineering. Discov Nano. 2024;19(1):125.
[6] LAU KYC, AMADEI G, ZERNICKA-GOETZ M. Assembly of complete mouse embryo models from embryonic and induced stem cell types in vitro. Nat Protoc. 2023;18(12):3662-3689.
[7] ROHRINGER S, GRASL C, EHRMANN K, et al. Biodegradable, Self-Reinforcing Vascular Grafts for In Situ Tissue Engineering Approaches. Adv Healthc Mater. 2023;12(23):e2300520.
[8] XIE C, RITCHIE RP, HUANG H, et al. Smooth muscle cell differentiation in vitro: models and underlying molecular mechanisms. Arterioscler Thromb Vasc Biol. 2011;31(7):1485-1494.
[9] GU W, HONG X, LE BRAS A, et al. Smooth muscle cells differentiated from mesenchymal stem cells are regulated by microRNAs and suitable for vascular tissue grafts. J Biol Chem. 2018;293(21):8089-8102.
[10] LIU M, GOMEZ D. Smooth Muscle Cell Phenotypic Diversity. Arterioscler Thromb Vasc Biol. 2019;39(9):1715-1723.
[11] ZHANG J, WANG L, FU W, et al. Smooth muscle cell phenotypic diversity between dissected and unaffected thoracic aortic media. J Cardiovasc Surg (Torino). 2013;54(4):511-521.
[12] FRISMANTIENE A, PHILIPPOVA M, ERNE P, et al. Smooth muscle cell-driven vascular diseases and molecular mechanisms of VSMC plasticity. Cell Signal. 2018;52:48-64.
[13] NEFF LP, TILLMAN BW, YAZDANI SK, et al. Vascular smooth muscle enhances functionality of tissue-engineered blood vessels in vivo. J Vasc Surg. 2011;53(2):426-434.
[14] YIN Z, ZHANG J, SHEN Z, et al. Regulated vascular smooth muscle cell death in vascular diseases. Cell Prolif. 2024;57(11):e13688.
[15] SHIMIZU K, SUGIYAMA S, AIKAWA M, et al. Host bone-marrow cells are a source of donor intimal smooth- muscle-like cells in murine aortic transplant arteriopathy. Nat Med. 2001;7(6):738-741.
[16] MIZUNO H. The potential for treatment of skeletal muscle disorders with adipose-derived stem cells. Curr Stem Cell Res Ther. 2010;5(2): 133-136.
[17] TIAN Z, YU T, LIU J, et al. Introduction to stem cells. Prog Mol Biol Transl Sci. 2023;199:3-32.
[18] DE MORREE A, RANDO TA. Regulation of adult stem cell quiescence and its functions in the maintenance of tissue integrity. Nat Rev Mol Cell Biol. 2023;24(5):334-354.
[19] LIU B, QU J, ZHANG W, et al. A stem cell aging framework, from mechanisms to interventions. Cell Rep. 2022;41(3):111451.
[20] MENS MMJ, GHANBARI M. Cell Cycle Regulation of Stem Cells by MicroRNAs. Stem Cell Rev Rep. 2018;14(3):309-322.
[21] PLANAT-BENARD V, SILVESTRE JS, COUSIN B, et al. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004; 109(5):656-663.
[22] WANG M, LIN S, MEQUANINT K. Electrospun Biodegradable α-Amino Acid-Substituted Poly(organophosphazene) Fiber Mats for Stem Cell Differentiation towards Vascular Smooth Muscle Cells. Polymers (Basel). 2022;14(8):1555.
[23] EL-NASEERY NI, ELEWA YHA, EL-BEHERY EI, et al. Human umbilical cord blood-derived mesenchymal stem cells restored hematopoiesis by improving radiation induced bone marrow niche remodeling in rats. Ann Anat. 2023;250:152131.
[24] KHORASANI HR, SANCHOULI M, MEHRANI J, et al. Potential of Bone-Marrow-Derived Mesenchymal Stem Cells for Maxillofacial and Periodontal Regeneration: A Narrative Review. Int J Dent. 2021; 2021:4759492.
[25] LIEN SC, USAMI S, CHIEN S, et al. Phosphatidylinositol 3-kinase/Akt pathway is involved in transforming growth factor-beta1-induced phenotypic modulation of 10T1/2 cells to smooth muscle cells. Cell Signal. 2006;18(8):1270-1278.
[26] IBIKUNLE S, GROSSO D, GERGIS U. The two-step approach to allogeneic hematopoietic stem cell transplantation. Front Immunol. 2023;14:1237782.
[27] BURT RK, ALEXANDER T, EBMT AUTOIMMUNE DISEASES WORKING PARTY (ADWP). Hematopoietic stem cell transplantation for multiple sclerosis: no inflammation, no response. Eur J Neurol. 2025;32(1):e16565.
[28] MARIOTTINI A, DE MATTEIS E, CENCIONI MT, et al. Haematopoietic Stem Cell Transplantation for the Treatment of Multiple Sclerosis: Recent Advances. Curr Neurol Neurosci Rep. 2023;23(9):507-520.
[29] ZHOU R, ZHU L, FU S, et al. Small Diameter Blood Vessels Bioengineered From Human Adipose-derived Stem Cells. Sci Rep. 2016;6:35422.
[30] DAVIS JP, SALMON M, POPE NH, et al. Attenuation of aortic aneurysms with stem cells from different genders. J Surg Res. 2015;199(1): 249-258.
[31] BARRIGA F, RAMÍREZ P, WIETSTRUCK A, et al. Hematopoietic stem cell transplantation: clinical use and perspectives. Biol Res. 2012;45(3): 307-316.
[32] LAROYE C, GAUTHIER M, ANTONOT H, et al. Mesenchymal Stem/Stromal Cell Production Compliant with Good Manufacturing Practice: Comparison between Bone Marrow, the Gold Standard Adult Source, and Wharton’s Jelly, an Extraembryonic Source. J Clin Med. 2019;8(12):2207.
[33] DI TRAPANI M, BASSI G, RICCIARDI M, et al. Comparative study of immune regulatory properties of stem cells derived from different tissues. Stem Cells Dev. 2013;22(22):2990-3002.
[34] MUNARIN F, KANT RJ, RUPERT CE, et al. Engineered human myocardium with local release of angiogenic proteins improves vascularization and cardiac function in injured rat hearts. Biomaterials. 2020;251:120033.
[35] ROY S, SCHMUCK E, RAVAL A. Macrophage Response to Biomaterials in Cardiovascular Applications. Stem Cells. 2021:81-92. doi: 10.1007/978-3-030-77052-5_6
[36] OLINIC M, LAZAR FL, ONEA HL, et al. Peripheral Artery Disease Ultrasound Assessment in Predicting the Severity of Coronary Artery Disease. Life (Basel). 2024;14(3):333.
[37] LEE J, GILLILAND TC, DRON J, et al. Integrative Metabolomics Differentiate Coronary Artery Disease, Peripheral Artery Disease, and Venous Thromboembolism Risks. Arterioscler Thromb Vasc Biol. 2024;44(9):2108-2117.
[38] ZHANG F, KING MW. Immunomodulation Strategies for the Successful Regeneration of a Tissue-Engineered Vascular Graft. Adv Healthc Mater. 2022;11(12):e2200045.
[39] SONG X, FU Y, LI C, et al. Single-cell RNA sequencing atlas of peripheral blood mononuclear cells from subjects with coronary artery disease. Biochim Biophys Acta Mol Cell Res. 2024;1871(1):119593.
[40] SONG HG, RUMMA RT, OZAKI CK, et al. Vascular Tissue Engineering: Progress, Challenges, and Clinical Promise. Cell Stem Cell. 2018; 22(3):340-354.
[41] SHI J, YANG Y, CHENG A, et al. Metabolism of vascular smooth muscle cells in vascular diseases. Am J Physiol Heart Circ Physiol. 2020;319(3):H613-H631.
[42] OWENS GK, KUMAR MS, WAMHOFF BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84(3):767-801.
[43] BROZOVICH FV, NICHOLSON CJ, DEGEN CV, et al. Mechanisms of Vascular Smooth Muscle Contraction and the Basis for Pharmacologic Treatment of Smooth Muscle Disorders. Pharmacol Rev. 2016;68(2): 476-532.
[44] WANG W, LIU D, LI D, et al. Nanofibrous vascular scaffold prepared from miscible polymer blend with heparin/stromal cell-derived factor-1 alpha for enhancing anticoagulation and endothelialization. Colloids Surf B Biointerfaces. 2019;181:963-972.
[45] WANG C, CEN L, YIN S, et al. A small diameter elastic blood vessel wall prepared under pulsatile conditions from polyglycolic acid mesh and smooth muscle cells differentiated from adipose-derived stem cells. Biomaterials. 2010;31(4):621-630.
[46] ZHANG X, BATTISTON KG, LABOW RS, et al. Generating favorable growth factor and protease release profiles to enable extracellular matrix accumulation within an in vitro tissue engineering environment. Acta Biomater. 2017;54:81-94.
[47] BERNSTEIN SR, KELLEHER C, KHALIL RA. Gender-based research underscores sex differences in biological processes, clinical disorders and pharmacological interventions. Biochem Pharmacol. 2023;215:115737.
[48] EDVINSSON JCA, GRUBOR I, MADDAHI A, et al. Male-female comparison of vasomotor effects of circulating hormones in human intracranial arteries. J Headache Pain. 2024;25(1):216. |