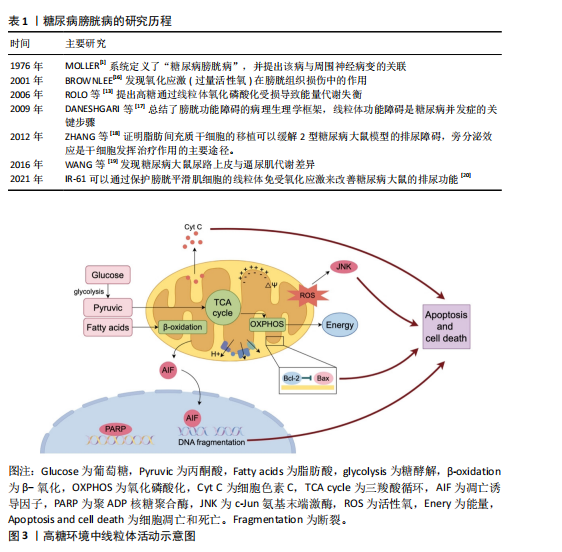
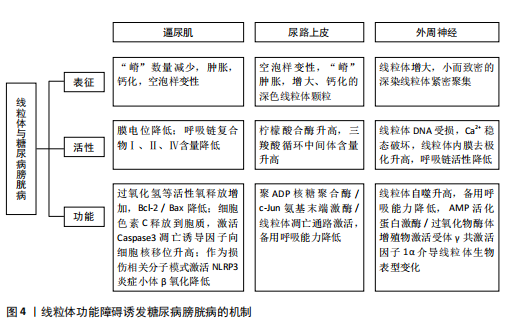
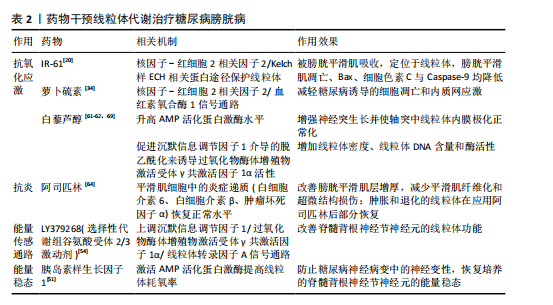
[1] MOLLER CF. Diabetic cystopathy.I: A clinical study of the frequency of bladder dysfunction in diabetics. Dan Med Bull. 1976;23(6):267-278.
[2] KWON MH, CHOI MJ, LIU FY, et al. Functional and immunofluorescence evaluations of vascular and neural integrities in urinary bladder of streptozotocin-induced diabetic mice. Int Neurourol J. 2022;26(3):201-209.
[3] YUAN Z, TANG Z, HE C, et al. Diabetic cystopathy: A review. J Diabetes. 2015;7(4): 442-447.
[4] BURNS RT, ARNOLD PJ, SONG L, et al. An analysis of urodynamic parameters in diabetic and nondiabetic women. Neurourol Urodyn. 2024;43(7):1600-1608.
[5] YANG XF, WANG J, RUI-WANG, et al. Time-dependent functional, morphological, and molecular changes in diabetic bladder dysfunction in streptozotocin-induced diabetic mice. Neurourol Urodyn. 2019;38(5):1266-1277.
[6] WANG J, REN L, LIU X, et al. Underactive Bladder and Detrusor Underactivity: New Advances and Prospectives. Int J Mol Sci. 2023;24(21):15517.
[7] WEI W, JIANG W, HAN T, et al. The future of prevention and treatment of diabetes with nutrition in China. Cell Metab. 2021; 33(10):1908-1910.
[8] JOSEPHINE MF, MARK EC. Mechanisms of diabetic complications. Physiol Rev. 2013;93(1):137-188.
[9] OLIVEIRA AL, DE OLIVEIRA MG, MONICA FZ, et al. Methylglyoxal and advanced glycation end products (AGEs): Targets for the prevention and treatment of diabetes-associated bladder dysfunction? Biomedicines. 2024;12(5):939.
[10] KALLINIKAS G, HARONIS G, KALLINIKA E, et al. A brief overview of cholinergic and phosphodiesterase-5 inhibitors in diabetic bladder dysfunction. Int J Mol Sci. 2024;25(19):10704.
[11] RYU CM, KIM Y, SHIN JH, et al. Mesenchymal stem cells with an enhanced antioxidant capacity integrate as smooth muscle cells in a model of diabetic detrusor underactivity. Clin Transl Med. 2024;14(10):e70052.
[12] MICHAEL B. The pathobiology of diabetic complications: A unifying mechanism. Diabetes. 2005;54(6):1615-1625.
[13] ROLO AP, PALMEIRA CM. Diabetes and mitochondrial function: Role of hyperglycemia and oxidative stress. Toxicol Appl Pharmacol. 2006;212(2):167-178.
[14] ZHANG L, WEI Y, YUAN S, et al. Targeting Mitochondrial Metabolic Reprogramming as a Potential Approach for Cancer Therapy. Int J Mol Sci. 2023;24(5):4954.
[15] ZHOU R, YAZDI AS, MENU P, et al. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469(7329): 221-225.
[16] BROWNLEE M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813-820.
[17] DANESHGARI F, LIU G, BIRDER L, et al. Diabetic bladder dysfunction: current translational knowledge. J Urol. 2009;182 (6 Suppl):S18-26.
[18] ZHANG H, QIU X, SHINDEL AW, et al. Adipose tissue-derived stem cells ameliorate diabetic bladder dysfunction in a type II diabetic rat model. Stem Cells Dev. 2012;21(9):1391-1400.
[19] WANG Y, DENG GG, DAVIES KD. Novel insights into development of diabetic bladder disorder provided by metabolomic analysis of the rat nondiabetic and diabetic detrusor and urothelial layer. Am J Physiol Endocrinol Metab. 2016;311(2):E471-E479.
[20] WANG J, DAI L, YUE X, et al. IR-61 Improves Voiding Function via Mitochondrial Protection in Diabetic Rats. Front Pharmacol. 2021;12:608637.
[21] MONZEL AS, ENRÍQUEZ JA, PICARD M. Multifaceted mitochondria: moving mitochondrial science beyond function and dysfunction. Nat Metab. 2023;5(4):546-562.
[22] YANG K, WANG Q. 50 week ultrasound imaging and ultrastructural abnormalities of bladder after sugar diuresis and diabetes mellitus in rats. Int Urol Nephrol. 2021;53(10):1995-2005.
[23] ROVIRA-LLOPIS S, BAÑULS C, DIAZ-MORALES N, et al. Mitochondrial dynamics in type 2 diabetes: Pathophysiological implications. Redox Biol. 2017;11:637-645.
[24] WESTERMANN B. Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol. 2010;11(12):872-884.
[25] ROVIRA-LLOPIS S, APOSTOLOVA N, BAÑULS C, et al. Mitochondria, the NLRP3 Inflammasome, and Sirtuins in Type 2 Diabetes: New Therapeutic Targets. Antioxid Redox Signal. 2018;29(8):749-791.
[26] RANIA AE, GUIMING L. Long-term diabetes causes molecular alterations related to fibrosis and apoptosis in rat urinary bladder. Exp Mol Pathol. 2019;111:104304.
[27] HAN X, GAO Y, YIN X, et al. Effect of Electroacupuncture on Bladder Dysfunction via Regulation of MLC and MLCK Phosphorylation in a Rat Model of Type 2 Diabetes Mellitus. Evid Based Complement Alternat Med. 2021;2021:5558890.
[28] KIRSCHNER-HERMANNS R, DANESHGARI F, VAHABI B, et al. Does diabetes mellitus-induced bladder remodeling affect lower urinary tract function? ICI-RS 2011. Neurourol Urodyn. 2012;31(3):359-364.
[29] POWERS SA, RYAN TE, PAK ES, et al. Chronic high-fat diet decreased detrusor mitochondrial respiration and increased nerve-mediated contractions. Neurourol Urodyn. 2019;38(6):1524-1532.
[30] SONG QX, SUN Y, DENG K, et al. Potential role of oxidative stress in the pathogenesis of diabetic bladder dysfunction. Nat Rev Urol. 2022;19(10):581-596.
[31] KLEE NS, MCCARTHY CG, LEWIS S, et al. Urothelial Senescence in the Pathophysiology of Diabetic Bladder Dysfunction—A Novel Hypothesis. Front Surg. 2018;5:72.
[32] KSIAZEK K, PASSOS JF, OLIJSLAGERS S, et al. Mitochondrial dysfunction is a possible cause of accelerated senescence of mesothelial cells exposed to high glucose. Biochem Biophys Res Commun. 2008;366(3):793-799.
[33] PATTI ME, CORVERA S. The Role of Mitochondria in the Pathogenesis of Type 2 Diabetes. Endocr Rev. 2010;31(3):364-395.
[34] LIN C, CHUEH TH, CHUNG CH, et al. Sulforaphane improves voiding function via the preserving mitochondrial function in diabetic rats. J Formos Med Assoc. 2020;119(9):1422-1430.
[35] WANG L, SUN W, REN G, et al. Deletion of Nrf2 induced severe oxidative stress and apoptosis in mice model of diabetic bladder dysfunction. Int Urol Nephrol. 2024;56(10):3231-3240.
[36] ELRASHIDY RA, KAVRAN M, ASKER ME, et al. Smooth muscle-specific deletion of MnSOD exacerbates diabetes-induced bladder dysfunction in mice. Am J Physiol Renal Physiol. 2019;317(4):F906-F912.
[37] MATHIEU J, FLEXOR M, LANOTTE M, et al. A PARP-1/JNK1 cascade participates in the synergistic apoptotic effect of TNFalpha and all-trans retinoic acid in APL cells. Oncogene. 2008;27(24):3361-3370.
[38] HUGHES FM JR, ODOM MR, CERVANTES A, et al. Inflammation triggered by the NLRP3 inflammasome is a critical driver of diabetic bladder dysfunction. Front Physiol. 2022;13:920487.
[39] HUGHES FM JR, HIRSHMAN NA, INOUYE BM, et al. NLRP3 Promotes Diabetic Bladder Dysfunction and Changes in Symptom-Specific Bladder Innervation. Diabetes. 2019;68(2):430-440.
[40] BIRDER L, ANDERSSON KE. Urothelial Signaling. Physiol Rev. 2013;93(2):653-680.
[41] RIZK DE, PADMANABHAN RK, TARIQ S, et al. Ultra-structural morphological abnormalities of the urinary bladder in streptozotocin-induced diabetic female rats. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17(2):143-154.
[42] LV B, CHEN T, XU Z, et al. Crocin protects retinal ganglion cells against H2O2-induced damage through the mitochondrial pathway and activation of NF-κB. Int J Mol Med. 2016;37(1):225-232.
[43] LI W, OH S. Diabetic cystopathy is associated with PARP/JNK/mitochondrial apoptotic pathway-mediated bladder apoptosis. Neurourol Urodyn. 2010;29(7):1332-1337.
[44] KOŞAN M, HAFEZ G, OZTÜRK B, et al. Effect of urothelium on bladder contractility in diabetic rats. Int J Urol. 2005;12(7):677-682.
[45] MUSSI N, STUARD WL, SANCHES JM, et al. Chronic Hyperglycemia Compromises Mitochondrial Function in Corneal Epithelial Cells: Implications for the Diabetic Cornea. Cells. 2022;11(16):2567.
[46] FRIMODT-MØLLER C. Diabetic cystopathy: epidemiology and related disorders. Ann Intern Med. 1980;92(2 Pt 2):318-321.
[47] ROY HA, GREEN AL. The central autonomic network and regulation of bladder function. Front Neurosci. 2019;13:535.
[48] INMAN DM, HARUN-OR-RASHID M. Metabolic vulnerability in the neurodegenerative disease glaucoma. Front Neurosci. 2017;11:146.
[49] GREENE DA, WINEGRAD AI. In vitro studies of the substrates for energy production and the effects of insulin on glucose utilization in the neural components of peripheral nerve. Diabetes. 1979;28(10):878-887.
[50] SAS KM, KAYAMPILLY P, BYUN J, et al. Tissue-specific metabolic reprogramming drives nutrient flux in diabetic complications. JCI Insight. 2016;1(15):e86976.
[51] MAGHANOORI MR, MARGULETS V, SMITH DR, et al. Sensory neurons derived from diabetic rats exhibit deficits in functional glycolysis and ATP that are ameliorated by IGF-1. Mol Metab. 2021;49:101191.
[52] RODRIGUEZ YA, KAUR S, NOLTE E, et al. Novologue Therapy Requires Heat Shock Protein 70 and Thioredoxin-Interacting Protein to Improve Mitochondrial Bioenergetics and Decrease Mitophagy in Diabetic Sensory Neurons. ACS Chem Neurosci. 2021;12(16):3049-3059.
[53] CHOWDHURY SK, SMITH DR, FERNYHOUGH P. The role of aberrant mitochondrial bioenergetics in diabetic neuropathy. Neurobiol Dis. 2013;51:56-65.
[54] CHANDRASEKARAN K, ANJANEYULU M, CHOI J, et al. Role of mitochondria in diabetic peripheral neuropathy: Influencing the NAD+-dependent SIRT1-PGC-1α-TFAM pathway. Int Rev Neurobiol. 2019;145:177-209.
[55] SCHMIDT RE, FENG D, WANG Q, et al. Effect of insulin and an erythropoietin-derived peptide (ARA290) on established neuritic dystrophy and neuronopathy in Akita (Ins2 Akita) diabetic mouse sympathetic ganglia. Exp Neurol. 2011;232(2):126-135.
[56] ADHYA P, SHARMA SS. Redox TRPs in diabetes and diabetic complications: Mechanisms and pharmacological modulation. Pharmacol Res. 2019;146:104271.
[57] LI F, MUNSEY ST, SIVAPRASADARAO A. TRPM2-mediated rise in mitochondrial Zn2+ promotes palmitate-induced mitochondrial fission and pancreatic β-cell death in rodents. Cell Death Differ. 2017;24(12):1999-2012.
[58] SHAROPOV RB, GULAK LK, PHILYPPOV BI, et al. TRPV1 alterations in urinary bladder dysfunction in a rat model of STZ-induced diabetes. Life Sci. 2018;193:207-213.
[59] KAHYA MC, NAZıROĞLU M, ÖVEY İS. Modulation of Diabetes-Induced Oxidative Stress, Apoptosis, and Ca2+ Entry Through TRPM2 and TRPV1 Channels in Dorsal Root Ganglion and Hippocampus of Diabetic Rats by Melatonin and Selenium. Mol Neurobiol. 2017;54(3):2345-2360.
[60] ERDOGAN BR, LIU G, ARIOGLU-INAN E, et al. Established and emerging treatments for diabetes-associated lower urinary tract dysfunction. Naunyn Schmiedebergs Arch Pharmacol. 2022;395(8):887-906.
[61] LAGOUGE M, ARGMANN C, GERHART-HINES Z, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127(6):1109-1122.
[62] REN B, KWAH M X-Y, LIU C, et al. Resveratrol for cancer therapy: Challenges and future perspectives. Cancer Lett. 2021;515:63-72.
[63] XU F, DU H, HOU J, et al. Anti-inflammation properties of resveratrol in the detrusor smooth muscle of the diabetic rat. Int Urol Nephrol. 2022;54(11):2833-2843.
[64] DU H, XU F, LIU J, et al. Long-term aspirin administration suppresses inflammation in diabetic cystopathy. Aging. 2023;15(17):9128-9143.
[65] CEN M, OUYANG W, ZHANG W, et al. MitoQ protects against hyperpermeability of endothelium barrier in acute lung injury via a Nrf2-dependent mechanism. Redox Biol. 2021;41:101936.
[66] 许洁,张思聪,高杰,等.干细胞移植治疗糖尿病膀胱的研究进展[J].现代泌尿外科杂志,2022,27(4):352-355,360.
[67] ISLAM MN, DAS SR, EMIN MT, et al. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18(5):759-765.
[68] KONARI N, NAGAISHI K, KIKUCHI S, et al. Mitochondria transfer from mesenchymal stem cells structurally and functionally repairs renal proximal tubular epithelial cells in diabetic nephropathy in vivo. Sci Rep. 2019;9(1):5184.
[69] ROY CHOWDHURY SK, SMITH DR, SALEH A, et al. Impaired adenosine monophosphate-activated protein kinase signalling in dorsal root ganglia neurons is linked to mitochondrial dysfunction and peripheral neuropathy in diabetes. Brain. 2012; 135(Pt 6):1751-1766.
[70] XUE B, KADEERHAN G, SUN LB, et al. Circulating exosomal miR-16-5p and let-7e-5p are associated with bladder fibrosis of diabetic cystopathy. Sci Rep. 2024;14(1): 837.
[71] 王书韵,谢君辉,余学锋.间充质干细胞治疗糖尿病肾病的作用与机制[J]. 中国组织工程研究,2022,26(1):148-152.
[72] YUAN Y, YUAN L, LI L, et al. Mitochondrial transfer from mesenchymal stem cells to macrophages restricts inflammation and alleviates kidney injury in diabetic nephropathy mice via PGC-1α activation. Stem Cells. 2021;39(7):913-928.
[73] PHINNEY DG, DI GIUSEPPE M, NJAH J, et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat Commun. 2015;6:8472.
[74] HINDI EA, WILLIAMS CJ, ZEEF LAH, et al. Experimental long-term diabetes mellitus alters the transcriptome and biomechanical properties of the rat urinary bladder. Sci Rep. 2021;11(1):15529.
|