[1] DIETRICHS E, HÅHEIM B, KONDRATIEV T, et al. Effects of hypothermia and rewarming on cardiovascular autonomic control in vivo. J Appl Phys. 2018;124:850-859.
[2] CHEN PGF, SUN Z. AAV Delivery of Endothelin-1 shRNA Attenuates Cold-Induced Hypertension. Human Gene Ther. 2017;28(2):190-199.
[3] GOEL H, SHAH K, KUMAR A, et al. Temperature, cardiovascular mortality, and the role of hypertension and renin-angiotensin-aldosterone axis in seasonal adversity: a narrative review. J Human Hypertens. 2022;36(12):1035-1047.
[4] XIONG S, WANG B, LIN S, et al. Activation of Transient Receptor Potential Melastatin Subtype 8 Attenuates Cold-Induced Hypertension Through Ameliorating Vascular Mitochondrial Dysfunction. J Am Heart Assoc. 2017;6(8):e005495.
[5] GONG J, LIU J, RONAN EA, et al. A Cold-Sensing Receptor Encoded by a Glutamate Receptor Gene. Cell. 2019;178(6):1375-1386.e11.
[6] CASTILLO K, DIAZ-FRANULIC I, CANAN J, et al. Thermally activated TRP channels: molecular sensors for temperature detection. Phys Biol. 2018;15(2):021001.
[7] YAMAKI S, CHAU A, GONZALES L, et al. Nociceptive afferent phenotyping reveals that transient receptor potential ankyrin 1 promotes cold pain through neurogenic inflammation upstream of the neurotrophic factor receptor GFRα3 and the menthol receptor transient receptor potential melastatin 8. Pain. 2021;162(2):609-618.
[8] LIU L, ROHACS T. Regulation of the cold-sensing TRPM8 channels by phosphoinositides and Gq-coupled receptors. Channels (Austin). 2020;14(1):79-86.
[9] LIN C, SHAN Y, WANG Z, et al. Molecular and circuit mechanisms underlying avoidance of rapid cooling stimuli in C. elegans. Nat Commun. 2024;15(1):297.
[10] MCKEMY D. The Molecular and Cellular Basis of Cold Sensation. ACS Chem Neurosci. 2013;4(2):238-47.
[11] WALTHER L, VON KÄNEL R, HEIMGARTNER N, et al. Altered Cardiovascular Reactivity to and Recovery from Cold Face Test-Induced Parasympathetic Stimulation in Essential Hypertension. J Clin Med. 2021; 10(12):2714.
[12] KANAYAMA N, KHATUN S, BELAYET H, et al. Chronic local cold stress to the soles induces hypertension in rats. Am J Hypertens. 1999; 12(11 Pt 1):1124-9.
[13] ROXANE B, CHANDROU K, ALEXANDRE C, et al. Gastrointestinal thermal homogeneity and effect of cold water ingestion. J Therm Biol. 2018;78:204-208.
[14] LIU Z, WU H, WEI Z, et al. TRPM8: a potential target for cancer treatment. J Cancer Res Clin Oncol. 2016;142(9):1871-1881.
[15] LUU D, RAMESH N, KAZAN I, et al. Evidence that the cold- and menthol-sensing functions of the human TRPM8 channel evolved separately. Sci Adv. 2024; 10(25):eadm9228.
[16] BLANQUART S, BOROWIEC A, DELCOURT P, et al. Evolution of the human cold/menthol receptor, TRPM8. Mol Phylogenet Evol. 2019;136:104-118.
[17] IFTINCA M, ALTIER C. The cool things to know about TRPM8! Channels (Austin). 2020;14(1):413-420.
[18] RADDATZ N, CASTILLO J, GONZALEZ C, et al. Temperature and Voltage Coupling to Channel Opening in Transient Receptor Potential Melastatin 8 (TRPM8). J Biol Chem. 2014;289(51):35438-35454.
[19] LIU B, FAN L, BALAKRISHNA S, et al. TRPM8 is the principal mediator of menthol-induced analgesia of acute and inflammatory pain. Pain. 2013;154(10):2169-2177.
[20] 李名伟,张淼,许玮华,等.瞬时受体电位8型的功能结构及其化学调节剂的研究进展[J].中国现代应用药学,2022, 39(19):2557-2566.
[21] YANG S, LU X, WANG Y, et al. A paradigm of thermal adaptation in penguins and elephants by tuning cold activation in TRPM8. Proc Natl Acad Sci U S A. 2020; 117(15):8633-8638.
[22] YARMOLINSKY DA, PENG Y, POGORZALA LA, et al. Coding and Plasticity in the Mammalian Thermosensory System. Neuron. 2016;92(5):1079-1092.
[23] PARICIO-MONTESINOS R, SCHWALLER F, UDHAYACHANDRAN A, et al. The Sensory Coding of Warm Perception. Neuron. 2020; 106(5):830-841.e3.
[24] GAVVA NR, SANDROCK R, ARNOLD GE, et al. Reduced TRPM8 expression underpins reduced migraine risk and attenuated cold pain sensation in humans. Sci Rep. 2019;9(1):19655.
[25] THAPA D, BARRETT B, ARGUNHAN F, et al. Influence of Cold-TRP Receptors on Cold-Influenced Behaviour. Pharmaceuticals(Basel). 2021;15(1):42.
[26] MELANAPHY D, JOHNSON C, KUSTOV M, et al. Ion channel mechanisms of rat tail artery contraction-relaxation by menthol involving, respectively, TRPM8 activation and L-type Ca2+ channel inhibition. Am J Physiol Heart Circ Physiol. 2016;311(6):H1416-H1430.
[27] CHEN X, XU L, ZHANG H, et al. Differential Activation of TRPM8 by the Stereoisomers of Menthol. Front Pharmacol. 2022;13: 898670.
[28] ORDAS P, HERNANDEZ-ORTEGO P, VARA H, et al. Expression of the cold thermoreceptor TRPM8 in rodent brain thermoregulatory circuits. J Comp Neurol. 2021;529(1):234-256.
[29] 袁源, 叶鹏, Wang Q, 等.食用薄荷醇通过瞬时受体电位M8缓解心肌梗死后炎症及心肌重塑[J].中华高血压杂志,2021, 29(1):98.
[30] RIVERA B, CAMPOS M, ORIO P, et al. Negative Modulation of TRPM8 Channel Function by Protein Kinase C in Trigeminal Cold Thermoreceptor Neurons. Int J Mol Sci. 2020;21(12):4420.
[31] IZQUIERDO C, MARTIN-MARTINEZ M, GOMEZ-MONTERREY I, et al. TRPM8 Channels: Advances in Structural Studies and Pharmacological Modulation. Int J Mol Sci. 2021;22(16):8502.
[32] CAI W, ZHANG W, ZHENG Q, et al. The kainate receptor GluK2 mediates cold sensing in mice. Nat Neurosci. 2024;27(4): 679-688.
[33] IIDA I, KONNO K, NATSUME R, et al. A comparative analysis of kainate receptor GluK2 and GluK5 knockout mice in a pure genetic background. Behav Brain Res. 2021; 405:113194.
[34] JOSEPH DJ, WILLIAMS DJ, MACDERMOTT AB. Modulation of Neurite Outgrowth by Activation of Calcium-Permeable Kainate Receptors Expressed by Rat Nociceptive-Like Dorsal Root Ganglion Neurons. Dev Neurobiol. 2011;71(10):818-835.
[35] BUIJS TJ, MCNAUGHTON PA. The Role of Cold-Sensitive Ion Channels in Peripheral Thermosensation. Front Cell Neurosci. 2020;14:262.
[36] LI YJ, DUAN GF, SUN JH, et al. Neto proteins regulate gating of the kainate-type glutamate receptor GluK2 through two binding sites. J Biol Chem. 2019;294(47):17889-17902.
[37] WANG Y, QIN J, SHARMA A, et al. Exploring the promise of regulator of G Protein Signaling 20: insights into potential mechanisms and prospects across solid cancers and hematological malignancies. Cancer Cell Int. 2024;24(1):305.
[38] TRAYNELIS S, WOLLMUTH L, MCBAIN C, et al. Glutamate Receptor Ion Channels: Structure, Regulation, and Function. Pharmacol Rev. 2010;62(3):405-496.
[39] TAKAYAMA Y, TOMINAGA M. Interaction between TRP channels and anoctamins. Cell Calcium. 2024;121:102912.
[40] DHAKAL S, LEE Y. Transient Receptor Potential Channels and Metabolism. Mol Cells. 2019;42(8):569-578.
[41] SINICA V, ZIMOVA L, BARVIKOVA K, et al. Human and Mouse TRPA1 Are Heat and Cold Sensors Differentially Tuned by Voltage. Cells. 2019;9(1):57.
[42] BABES A, CIOBANU AC, NEACSU C, et al. TRPM8, a Sensor for Mild Cooling in Mammalian Sensory Nerve Endings. Curr Pharm Biotechnol. 2011;12(1):78-88.
[43] CAI W, DUAN B, XU XZS. Identification of a cold sensor in peripheral somatosensory neurons. Nat Neurosci. 2024;27(4):613-614.
[44] SHARMA N, FLAHERTY K, LEZGIYEVA K, et al. The emergence of transcriptional identity in somatosensory neurons. Nature. 2020;577(7790):392-398.
[45] PERTUSA M, GONZALEZ A, HARDY P, et al. Bidirectional Modulation of Thermal and Chemical Sensitivity of TRPM8 Channels by the Initial Region of the N-terminal Domain. J Biol Chem. 2014;289(32):21828-21843.
[46] DE CARO C, CRISTIANO C, AVAGLIANO C, et al. Characterization of New TRPM8 Modulators in Pain Perception. Int J Mol Sci. 2019;20(22):5544.
[47] GKIKA D, LOLIGNIER S, GROLEZ GP, et al. Testosterone-androgen receptor: The steroid link inhibiting TRPM8-mediated cold sensitivity. FASEB J. 2020;34(6):7483-7499.
[48] KASUGA R, SHIRAKI C, HORIKAWA R, et al. Role of TRPM8 in cold avoidance behaviors and brain activation during innocuous and nocuous cold stimuli. Physiol Behav. 2022;248:113729.
[49] PIÑA R, UGARTE G, CAMPOS M, et al. Role of TRPM8 Channels in Altered Cold Sensitivity of Corneal Primary Sensory Neurons Induced by Axonal Damage. J Neurosci. 2019;39(41):8177-8192.
[50] LING Y, CHEN S, FANN C, et al. TRPM8 genetic variant is associated with chronic migraine and allodynia. J Headache Pain. 2019;20(1):115.
[51] KOBAYASHI M, ZOCHODNE D. Diabetic neuropathy and the sensory neuron: New aspects of pathogenesis and their treatment implications. J Diabetes Investig. 2018;9(6):1239-1254.
[52] HOSSAIN M, ANDO H, UNNO S, et al. Activation of TRPV1 and TRPM8 Channels in the Larynx and Associated Laryngopharyngeal Regions Facilitates the Swallowing Reflex. Int J Mol Sci. 2018; 19(12):4113.
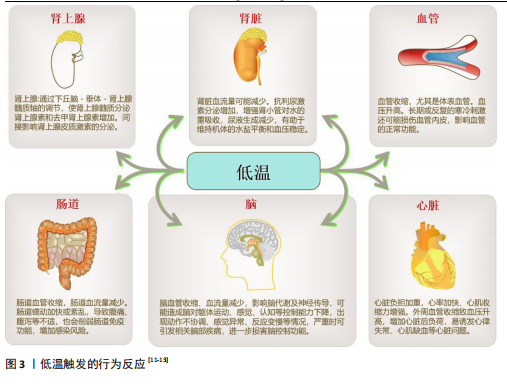
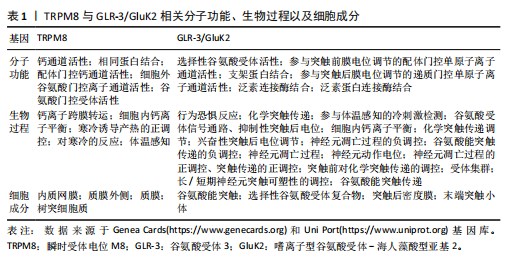
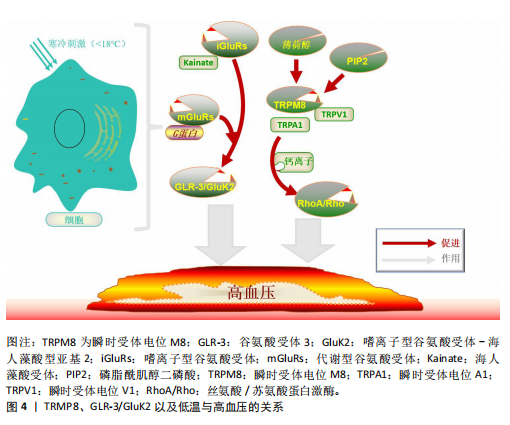
[53] DAS UN. Potential Role of TRPM8 in Cold-Induced Hypertension and Its Clinical Implications. Discov Med. 2023; 35(177):451-457.
[54] ZHANG Z, ZHAO L, ZHOU X, et al. Role of inflammation, immunity, and oxidative stress in hypertension: New insights and potential therapeutic targets. Front Immunol. 2023;13:1098725.
[55] HUANG F, NI M, ZHANG JM, et al. TRPM8 downregulation by angiotensin II in vascular smooth muscle cells is involved in hypertension. Mol Med Rep. 2017;15(4): 1900-1908.
[56] CHAN H, HUANG HS, SUN DS, et al. TRPM8 and RAAS-mediated hypertension is critical for cold-induced immunosuppression in mice. Oncotarget. 2018;9(16):12781-12795.
[57] DE JONG PR, TAKAHASHI N, PEIRIS M, et al. TRPM8 on mucosal sensory nerves regulates colitogenic responses by innate immune cells via CGRP. Mucosal Immunol. 2015;8(3):491-504.
[58] WANG Q, YANG Y, CHEN K, et al. Dietary Menthol Attenuates Inflammation and Cardiac Remodeling After Myocardial Infarction via the Transient Receptor Potential Melastatin 8. Am J Hypertens. 2020;33(3):223-233.
[59] EGBENYA D, AIDOO E, KYEI G. Glutamate receptors in brain development. Childs Nerv Syst. 2021;37(9):2753-2758.
[60] RAMOS-VICENTE D, GRANT S, BAYÉS A. Metazoan evolution and diversity of glutamate receptors and their auxiliary subunits. Neuropharmacology. 2021;195: 108640.
[61] LI DP, PAN HL. Glutamatergic Regulation of Hypothalamic Presympathetic Neurons in Hypertension. Curr Hypertens Rep. 2017; 19(10):78.
[62] 尹桂东,崔桂玉,邴艳华,等.下丘脑室旁核的I组促代谢型谷氨酸受体在压力感受性反射调节中的作用[J].中国现代医学杂志,2010,20(10):1477-1481.
[63] ZHOU JJ, PACHUAU J, LI DP, et al. Group III metabotropic glutamate receptors regulate hypothalamic presympathetic neurons through opposing presynaptic and postsynaptic actions in hypertension. Neuropharmacology. 2020;174:108159.
[64] LI DP, PAN HL. Increased group I metabotropic glutamate receptor activity in paraventricular nucleus supports elevated sympathetic vasomotor tone in hypertension. Am J Physiol Regul Integr Comp Physiol. 2010;299(2):R552-R561.
[65] HSU J, SEKIZAWA S, TOCHINAI R, et al. Loss of Group II Metabotropic Glutamate Receptor Signaling Exacerbates Hypertension in Spontaneously Hypertensive Rats. Life (Basel). 2021;11(7):720.
[66] LI DP, BYAN HS, PAN HL. Switch to Glutamate Receptor 2-Lacking AMPA Receptors Increases Neuronal Excitability in Hypothalamus and Sympathetic Drive in Hypertension. J Neurosci. 2012;32(1):372-380.
[67] 谢金燕,洪葵. NMDA受体及其与心血管疾病关系的研究进展[J].中南大学学报(医学版),2017,42(11):1316-1320.
[68] HSU JCN, SEKIZAWA SI, TOCHINAI R, et al. Chronic stimulation of group II metabotropic glutamate receptors in the medulla oblongata attenuates hypertension development in spontaneously hypertensive rats. PLoS One. 2021;16(5):e0251495.
[69] HERMES SM, MITCHELL JL, SILVERMAN MB, et al. Sustained hypertension increases the density of AMPA receptor subunit, GluR1, in baroreceptive regions of the nucleus tractus solitarii of the rat. Brain Res. 2008;1187:125-136.
[70] ZHOU X, YANG H, SONG X, et al. Central blockade of the AT1 receptor attenuates pressor effects via reduction of glutamate release and downregulation of NMDA/AMPA receptors in the rostral ventrolateral medulla of rats with stress-induced hypertension. Hypertens Res. 2019;42(8): 1142-1151.
[71] BURADA A, VINNAKOTA R, BHARTI P, et al. Emerging insights into the structure and function of ionotropic glutamate delta receptors. Br J Pharmacol. 2022;179(14): 3612-3627.
[72] GANGWAR SP, YELSHANSKAYA MV, NADEZHDIN KD, et al. Kainate receptor channel opening and gating mechanism. Nature. 2024;630(8017):762-768.
[73] HE L, SUN J, GAO Y, et al. Kainate receptor modulation by NETO2. Nature. 2021;599(7884):325-329.
[74] NAIR JD, WILKINSON KA, YUCEL BP, et al. GluK2 Q/R editing regulates kainate receptor signaling and long-term potentiation of AMPA receptors. iScience. 2023;26(10):107708.
[75] THOMPSON K, WATSON S, ZANATO C, et al. The atypical hippocampal’ glutamate receptor coupled to phospholipase D that controls stretch-sensitivity in primary mechanosensory nerve endings is homomeric purely metabotropic GluK2. Exp Physiol. 2024;109(1):81-99.
[76] GORLEWICZ A, BARTHET G, ZUCCA S, et al. The Deletion of GluK2 Alters Cholinergic Control of Neuronal Excitability. Cereb Cortex. 2022;32(14):2907-2923.
[77] REGONI M, CATTANEO S, MERCATELLI D, et al. Pharmacological antagonism of kainate receptor rescues dysfunction and loss of dopamine neurons in a mouse model of human parkin-induced toxicity. Cell Death Dis. 2020;11(11):963.
[78] WANG L, LIU Y, LU R, et al. The role of S-nitrosylation of kainate-type of ionotropic glutamate receptor 2 in epilepsy induced by kainic acid. J Neurochem. 2018;144(3):255-270.
[79] PENG L, LI B, DU T, et al. Does conventional anti-bipolar and antidepressant drug therapy reduce NMDA-mediated neuronal excitation by downregulating astrocytic GluK2 function? Pharmacol Biochem Behav. 2012;100(4):712-725.
[80] DE VITA A, BELMUSTO A, DI PERNA F, et al. The Impact of Climate Change and Extreme Weather Conditions on Cardiovascular Health and Acute Cardiovascular Diseases. J Clin Med. 2024;13(3):759.
[81] POLDERMAN KH, VARON J. Cool hemodynamics - The intricate interplay between therapeutic hypothermia and the post-cardiac arrest syndrome. Resuscitation. 2014;85(8):975-976.
[82] 顾恺, 何红.高血压患者夏季和冬季动态血压监测值的差异[J].中华高血压杂志, 2016, 24(6):578-580.
[83] SUN Z. Cardiovascular responses to cold exposure. Front Biosci (Elite Ed). 2010;2(2): 495-503.
[84] ALBA BK, CASTELLANI JW, CHARKOUDIAN N. Cold-induced cutaneous vasoconstriction in humans: Function, dysfunction and the distinctly counterproductive. Exp Physiol. 2019;104(8):1202-1214.
[85] TARAPACKI P, JORGENSEN LB, SORENSEN JG, et al. Acclimation, duration and intensity of cold exposure determine the rate of cold stress accumulation and mortality in Drosophila suzukii. J Insect Physiol. 2021; 135:104323.
[86] KONG X, LIU H, HE X, et al. Unraveling the Mystery of Cold Stress-Induced Myocardial Injury. Front Physiol. 2020;11:580811.
[87] DRYN DO, MELNYK MI, MELANAPHY D, et al. Bidirectional TRP/L Type Ca2+ Channel/RyR/BKCa Molecular and Functional Signaloplex in Vascular Smooth Muscles. Biomolecules. 2023;13(5):759.
[88] YE ZY, LI DP, PAN HL. Regulation of Hypothalamic Presympathetic Neurons and Sympathetic Outflow by Group II Metabotropic Glutamate Receptors in Spontaneously Hypertensive Rats. Hypertension. 2013;62(2):255-262.
[89] BURADA A, VINNAKOTA R, LAMBOLEZ B, et al. Structural biology of ionotropic glutamate delta receptors and their crosstalk with metabotropic glutamate receptors. Neuropharmacology. 2021;196:108683.
|