中国组织工程研究 ›› 2025, Vol. 29 ›› Issue (4): 827-838.doi: 10.12307/2024.811
• 生物材料综述 biomaterial review • 上一篇 下一篇
磁性纳米材料与磁场效应加速骨损伤修复
肖 放1,黄 雷1,王 琳1,2
- 华中科技大学同济医学院附属协和医院,1组织工程与再生医学研究中心,2检验科,湖北省武汉市 430022
-
收稿日期:2023-11-16接受日期:2024-01-26出版日期:2025-02-08发布日期:2024-06-03 -
通讯作者:王琳,教授,华中科技大学同济医学院附属协和医院,组织工程与再生医学研究中心,检验科,湖北省武汉市 430022 -
作者简介:肖放,1999年生,湖北省荆门市人,汉族,华中科技大学在读硕士,主要从事骨组织工程相关研究。
Magnetic nanomaterials and magnetic field effects accelerate bone injury repair
Xiao Fang1, Huang Lei1, Wang Lin1, 2
- 1Research Center of Tissue Engineering and Regenerative Medicine, 2Department of Laboratory Medicine, Union Hospital Affiliated to Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430022, Hubei Province, China
-
Received:2023-11-16Accepted:2024-01-26Online:2025-02-08Published:2024-06-03 -
Contact:Wang Lin, Professor, Research Center of Tissue Engineering and Regenerative Medicine, and Department of Laboratory Medicine, Union Hospital Affiliated to Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430022, Hubei Province, China -
About author:Xiao Fang, Maser candidate, Research Center of Tissue Engineering and Regenerative Medicine, Union Hospital Affiliated to Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430022, Hubei Province, China
摘要:
文题释义:
磁性纳米材料:是指结构尺寸在1-100 nm范围内的磁性材料,以及由它们为基本单元构成的磁性材料。磁场:是指传递实物间磁力作用的场。是一种看不见、摸不着,而又客观存在的特殊物质,对放入其中的磁性材料有磁力作用。
背景:磁性纳米材料具有促进干细胞成骨分化和抑制破骨细胞形成等生物活性,并在磁场协同下有效促进损伤骨组织愈合,在骨损伤修复中具有广阔的应用前景。
目的:综述磁性纳米材料与磁场促进骨修复的作用机制,及其在骨损伤修复领域的研究进展。
方法:在PubMed和Web of Science数据库中进行相关文献检索,检索词为“magnetic nanomaterials,magnetic field,bone repair,bone tissue engineering,stem cell,osteoblast,osteoclast”。检索文献时限为2003-2023年,将其进行筛选和分析,最终纳入98篇文献进行分析。
结果与结论:①磁性纳米材料具有促成骨细胞分化、抑制破骨细胞形成和调节免疫微环境等生物效应;此外,磁性纳米材料可调节组织工程支架的机械性能和表面形貌等理化性质,并赋予其磁性,有利于调控干细胞的黏附、增殖与成骨分化。②磁场具有调控细胞内多条信号通路发挥促成骨细胞分化、抑制破骨细胞形成和刺激血管生成等生物学效应,从而加速损伤的骨组织愈合。③磁性纳米材料与磁场的联合应用,通过激活机械转导、增加细胞内磁性纳米粒子含量、增强微磁场效应等加速骨损伤修复,为骨组织工程的研究提供了新的思路。④磁场在临床骨折、骨质疏松症和骨关节炎疾病的治疗中展现出确切疗效,促进骨组织生长、减轻骨质流失和缓解疼痛,改善患者的生活质量。⑤磁性纳米材料与磁场在骨损伤修复与再生中应用潜力大,但磁性纳米材料、磁场和细胞之间的相互作用机制尚未完全阐明,而且磁场调控细胞内分子事件的关键参数,包括磁场类型、强度、频率、作用时间和作用方式等,以及特定磁场对骨细胞的精确生物效应和潜在机制仍有待明确。⑥未来需要进一步关注其对损伤组织微环境中的破骨细胞、神经、血管和免疫细胞的影响,而关于磁性材料用于人体安全性问题,有待系统性研究其分布、代谢以及急性和慢性毒性等。
https://orcid.org/0009-0008-0579-2584(肖放);https://orcid.org/0000-0001-5716-6587(王琳)
中国组织工程研究杂志出版内容重点:生物材料;骨生物材料;口腔生物材料;纳米材料;缓释材料;材料相容性;组织工程
中图分类号:
引用本文
肖 放, 黄 雷, 王 琳. 磁性纳米材料与磁场效应加速骨损伤修复[J]. 中国组织工程研究, 2025, 29(4): 827-838.
Xiao Fang, Huang Lei, Wang Lin. Magnetic nanomaterials and magnetic field effects accelerate bone injury repair[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 827-838.
2.2 磁性纳米粒子促进骨损伤修复的方式 在无外加磁场下,磁性纳米粒子可直接调控细胞行为,尤其是对干细胞增殖以及分化的调节。同时,磁性纳米粒子还可以调节骨组织工程支架的理化性质和生物活性,促进骨组织损伤的修复[9-15]。
2.2.1 磁性纳米粒子调控细胞行为促进骨损伤修复 磁性纳米粒子可促进干细胞的增殖以及成骨分化,加速体内骨形成和骨缺损修复。早在2008年,PARETA等[7]就证实磷酸钙包被的γ-Fe2O3磁性纳米颗粒显著促进人成骨细胞增殖,增加成骨细胞密度。已有研究表明磁性纳米颗粒具有天然的过氧化物酶样活性[16],HUANG等[17]在此基础上,证明了超顺磁性氧化铁纳米粒子通过过氧化物酶作用,降低细胞内H2O2含量,从而促进人间充质干细胞的增殖。此外,超顺磁性氧化铁纳米粒子进入溶酶体后,发生降解释放的游离铁(Fe)加速细胞周期,并调节细胞周期蛋白质调节因子的表达,促进人间充质干细胞增殖[17]。TRAN等[18]发现羟基磷灰石包裹的Fe3O4纳米粒子提高了成骨细胞的碱性磷酸酶活性、促进更多的胶原蛋白合成和钙化。
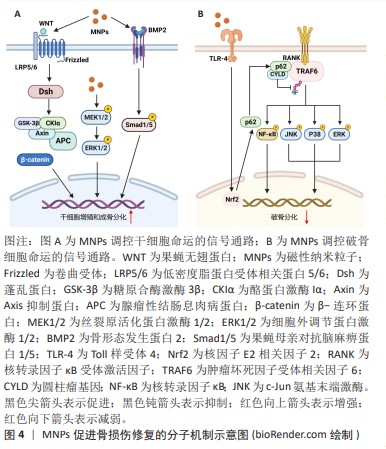
干细胞的成骨分化涉及多种信号通路和转录因子的激活,磁性纳米粒子通过调节成骨分化的关键信号途径,如Wnt信号通路、丝裂原活化蛋白激酶(mitogen-activated protein kinases,MAPK)信号通路以及骨形态发生蛋白/Smad信号通路[19-21],促进干细胞的成骨分化,见图4A。XIA等[19]研究发现添加了超顺磁性氧化铁纳米粒子的磷酸钙水泥支架促进了人牙髓干细胞的成骨分化,同时在基因和蛋白质水平上检测到上调的Wnt/β-Catenin信号,并且这种上调作用可被Wnt抑制剂DKK1减弱。WANG等[20]制备了多葡萄糖山梨醇羧甲基醚包被的超顺磁性氧化铁纳米粒子,研究其对人骨髓间充质干细胞成骨分化的影响,他们发现多葡萄糖山梨醇羧甲基醚包被的超顺磁性氧化铁纳米粒子激活了经典MAPK信号通路,并上调与成骨分化相关的下游基因,促进了人骨髓间充质干细胞的成骨分化。LU等[21]制备了磁性SrFe12O19纳米颗粒修饰的介孔生物玻璃/壳聚糖多孔支架用于骨再生治疗,研究表明该支架可以通过骨形态发生蛋白2/Smad/Runt相关转录因子2(runt Related transcription factor 2,Runx2)途径促进人骨髓间充质干细胞的增殖和成骨分化,促进骨损伤修复。
破骨细胞起源于造血干细胞,主要参与骨吸收,与成骨细胞协调维持正常的骨稳态和重塑,其活力上调可导致骨质疏松症。超顺磁性氧化铁纳米粒子具有调节破骨细胞分化的作用,见图4B。LIU等[22]发现临床常用的超顺磁性氧化铁纳米粒子(Ferumoxyto和Ferucarbotran)抑制核因子κB受体活化因子配体(receptor activator of nuclear factor-κB ligand,RANKL)诱导的骨髓来源单核/巨噬细胞的破骨细胞分化,并抑制RANKL诱导的肌动蛋白环的形成进而抑制破骨细胞的成熟。作者进一步发现超顺磁性氧化铁纳米粒子能上调骨髓来源单核/巨噬细胞p62的表达,招募圆柱瘤基因并增强肿瘤坏死因子受体相关因子6的去泛素化,抑制RANKL下游的MAPK和核转录因子κB信号通路,导致破骨相关基因水平的降低[22]。在此基础上,LI等[23]开发了一类具有核壳结构的羟基磷灰石包裹的超顺磁性氧化铁纳米颗粒,其可以促进间充质干细胞成骨分化并抑制骨髓来源单核/巨噬细胞的破骨细胞分化,显著降低了卵巢切除小鼠的骨质流失。然而,有研究报道过量的铁会激活破骨细胞导致骨质流失不利于骨组织的修复[24]。因此,在使用超顺磁性氧化铁纳米粒子治疗骨损伤时需控制铁的用量以减少其过度激活破骨细胞导致的负面影响。
总之,磁性纳米粒子可以直接调控细胞行为,促进干细胞增殖、成骨分化,并抑制破骨细胞形成,加速骨缺损修复并防止骨质流失。
2.2.2 磁性纳米粒子改善骨组织工程支架的生物性能促进骨损伤修复 细胞外基质调控细胞的行为和功能。其中,细胞外基质的物理信号,特别是基质刚度和形貌,是调控细胞行为的关键因素[25]。磁性纳米粒子通过改善细胞支架的理化性质,仿骨组织细胞外基质的生物活性,达到促进骨再生修复的目的。BIN等[26]将超顺磁性Fe3O4纳米颗粒掺入聚L-乳酸支架,增强了聚L-乳酸支架的力学性能和生物活性,该支架促进了人骨肉瘤细胞的黏附、扩散和相互作用,增加了碱性磷酸酶的活性,促进了细胞的增殖和成骨分化。XIA等[27]将超顺磁性氧化铁纳米粒子掺入磷酸钙水泥支架,发现支架上生长的人牙髓干细胞的成骨分化显著增强,与没有超顺磁性氧化铁纳米粒子的支架相比,人牙髓干细胞的碱性磷酸酶活性和成骨基因表达显著增加,骨基质矿物质增加两三倍。作者推测超顺磁性氧化铁纳米粒子-磷酸钙水泥支架的表面纳米形貌促进了人牙髓干细胞的成骨分化。
生物材料表面吸附的蛋白质层(蛋白冠)影响其在体内的生物效应。超顺磁性氧化铁纳米粒子可与多种蛋白相互作用,从而影响植入生物体内的支架表面蛋白冠的组成,其中,包括具有调节骨损伤修复作用的蛋白质[28-29]。ZHU等[28]构建了一种简单有效的磁性羟基磷灰石支架蛋白冠体内动力学模型,发现在磁性羟基磷灰石支架的蛋白冠中与免疫应答、炎症、骨和伤口愈合、细胞外基质、细胞行为和信号传导相关的蛋白质以时间依赖的方式增加;存在于磁性羟基磷灰石支架表面的相关蛋白冠分子促进了免疫细胞的募集和细胞外基质的重塑,加速骨愈合。在另一项研究中,作者还发现超顺磁性氧化铁纳米粒子增强了磁性羟基磷灰石与细胞增殖相关的蛋白质的吸附[29],特别是与MAPK/细胞外调节蛋白激酶(extracellular regulated protein kinases,ERK)信号通路相关的蛋白质含量更高;阻断MAPK信号通路的实验结果进一步表明,超顺磁性氧化铁纳米粒子介导的蛋白冠激活MAPK/ERK信号通路,进而促进磁性羟基磷灰石支架诱导小鼠胚胎成骨细胞增殖。
总之,磁性纳米粒子可以改变支架的力学性能、表面纳米形貌和蛋白冠,赋予支架优异的促进骨修复性能。
2.2.3 磁性纳米粒子通过纳米尺度的磁场促进骨损伤修复 磁性纳米粒子自身是没有畴壁的单个磁畴,可作为微小磁源产生纳米尺度的磁场,在细胞和支架接触的界面处提供微运动,影响细胞膜上的离子通道并触发机械转导途径,调节细胞增殖与分化,促进骨组织再生与修复。BIN等[26]将超顺磁性氧化铁纳米粒子掺入通过选择性激光烧结制备的聚L-乳酸骨支架中,超顺磁性氧化铁纳米粒子提供的微小磁场连续稳定地对人骨肉瘤细胞产生磁刺激,促进细胞的增殖与成骨分化。CHEN等[30]将静电纺丝技术和逐层组装技术相结合制造了磁性聚乳酸羟基乙酸/聚己内酯支架,并采用相同的方法制备了金纳米颗粒组装的聚乳酸羟基乙酸/聚己内酯支架,结果表明,与金纳米颗粒组装支架相比,磁性纳米颗粒组装支架具有更优异的成骨能力,进一步研究表明磁性纳米粒子不仅改善了支架的力学性能,同时其介导的磁效应还促进了细胞的成骨分化。

2.3 磁场促进骨损伤修复的作用 磁场已被证实对骨折愈合、脊柱融合和骨关节炎具有确切的疗效。其中,静磁场和脉冲电磁场在基础及临床研究中应用最为广泛。
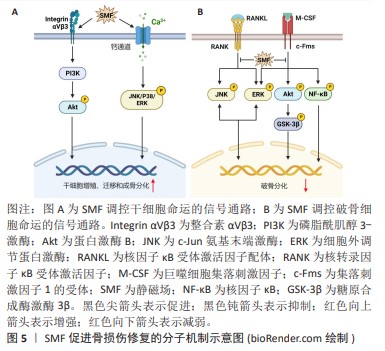
2.3.1 静磁场促进骨损伤修复 静磁场可以经磁铁块或电磁铁产生,相比于复杂的动磁场,具有设备简易且不良反应少等优点。静磁场可调节细胞迁移、增殖、细胞周期分布、凋亡和分化等,发挥促进新生骨形成抑制骨质流失的功能。ZHANG等[31]发现320 mT的静磁场可上调细胞增殖因子的表达水平,进而促进牙周韧带干细胞的增殖。在划痕试验中,静磁场处理的牙周韧带干细胞表现出更高的迁移速率,表明静磁场促进了牙周韧带干细胞的迁移能力。此外,暴露于静磁场的牙周韧带干细胞表现出更强的成骨分化能力,其碱性磷酸酶活性水平和基质矿化水平以及成骨细胞特异性基因和蛋白质表达均得到增强。同样地,骨髓间充质干细胞、脂肪衍生间充质干细胞、牙髓干细胞、小鼠胚胎成骨细胞等多种细胞在中等强度的静磁场刺激下表现出更强的增殖、迁移以及成骨分化活性[32-35]。NA等[34]报道1 mT的静磁场上调牙髓干细胞c-Jun氨基末端激酶(c-Jun N-terminal kinase,JNK),p38和ERK的磷酸化,激活MAPK信号通路,进而诱导牙髓干细胞增殖、迁移和成骨分化。WU等[32]证明MAPK信号通路的激活与T型钙离子通道的打开有关。MAREDZIAK等[33]观察到脂肪衍生间充质干细胞在500 mT的静磁场刺激下细胞活力增加,增殖加速;当使用磷脂酰肌醇3-激酶
(phosphatidylinositol 3-kinase,PI3K)抑制剂后,静磁场这种促细胞增殖作用消失。因此,静磁场可以通过MAPK和PI3K/丝氨酸/苏氨酸蛋白激酶B(serine/threonine protein kinase B,PKB或Akt)信号通路调节干细胞的增殖、迁移和成骨分化,见图5A。
与成骨细胞相比,静磁场对破骨细胞影响的研究较少。KIM等[36]发现15 mT的静磁场抑制RANKL诱导的破骨细胞分化的多条信号通路,包括Akt,糖原合酶激酶3β (glycogen synthase kinase 3β,GSK3β),MAPK和核转录因子κB信号通路,导致RANKL诱导的破骨细胞特异性转录因子(nuclear factor of activated T cells cytoplasmic 1,NFATc1)的表达显著下调,抑制小鼠骨髓来源单核/巨噬细胞分化为破骨细胞,降低破骨细胞抗酒石酸酸性磷酸酶活性和骨吸收能力,见图5B。ZHANG等[37]发现在16 T的静磁场作用下破骨细胞形成显著减少,破骨细胞分化相关基因的表达降低,而一氧化氮合酶活性增强,产生高水平的一氧化氮;当用一氧化氮合酶抑制剂处理时,破骨细胞的形成恢复到对照组水平。且研究提示升高的一氧化氮通过激活下游可溶性鸟苷酸环化酶(soluble guanylate cyclase,sGC)/环磷酸鸟苷(cyclic guanosine monophosphate,cGMP)/蛋白激酶G(protein kinase G,PKG)信号抑制破骨细胞的形成[38],表明静磁场增强破骨细胞中一氧化氮的合成抑制破骨细胞形成。此外,DONG等[39]发现,16 T的静磁场抑制破骨细胞形成和骨吸收能力可能与高磁场强度对细胞铁代谢的调节有关。研究表明过量的铁可以通过增加细胞中活性氧的产生,进而激活JNK,ERK和核转录因子κB信号通路促进破骨细胞的分化、增强骨吸收能力[40]。静磁场通过降低破骨细胞铁吸收和铁储存相关蛋白表达,提高铁输出相关蛋白表达,降低细胞内铁含量和抑制活性氧的产生,从而抑制破骨细胞生成和骨吸收活性[39]。因此,静磁场可能通过调节破骨细胞一氧化氮和铁代谢,抑制RANKL诱导的破骨细胞分化和骨吸收活性。
与脉冲电磁场相比,静磁场更易实现、并且没有热效应和电效应,因此更适合长期磁刺激。静磁场可以促进干细胞的增殖、迁移和成骨分化并抑制破骨细胞形成,然而其作用机制仍未完全阐明。
2.3.2 脉冲电磁场促进骨损伤修复 脉冲电磁场通过调控瞬时磁场频率、波形和峰值等参数,实现磁场的控制和调节,具有更为广泛的临床转化应用前景。脉冲电磁场通过触发Ca2+级联反应、骨形态发生蛋白/Smad和Wnt/β-Catenin等途径影响细胞多种生物学行为,具有促进新生骨生长、抑制破骨细胞活性和血管形成等生物效应。
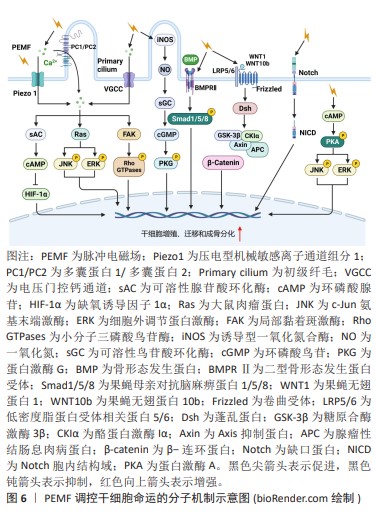
初级纤毛是感受脉冲电磁场刺激的主要细胞器,调控多条与成骨分化相关的信号通路,对成骨细胞分化不可或缺。YAN等[41]报道15 Hz/ 2 mT的脉冲电磁场激活成骨细胞初级纤毛上的多囊蛋白1/多囊蛋白2复合物,介导Ca2+内流并激活Ras/MAPK/激活蛋1(activator
protein-1,AP-1)轴,调节Ku70转录。Ku70识别、结合和桥接受损DNA以及维持细胞非同源末端连接复合物的稳定性,抑制细胞死亡。因此,脉冲电磁场上调放疗损伤的成骨细胞Ku70转录,促进其DNA修复与存活,最终显著抑制放疗诱导的骨质流失。在慢性低压缺氧条件下,缺氧诱导因子1α在成骨细胞中上调,与启动子中的缺氧反应元件结合,抑制成骨细胞分化相关分子Runx2和碱性磷酸酶的基因转录[42]。HAO等[42]发现脉冲电磁场刺激多囊蛋白1/多囊蛋白2,激活Ca2+/sAC/cAMP信号传导,抑制成骨细胞谱系中缺氧诱导因子1α表达,从而上调Runx2和碱性磷酸酶的转录,促进成骨细胞分化,有效抑制慢性高原缺氧引起的骨骼退化。此外,脉冲电磁场可通过激活电压门控钙通道和压电型机械敏感离子通道组分1(Piezo1)介导胞内Ca2+浓度升高[43-44]。ZHANG等[45]证明50 Hz/1 mT的脉冲电磁场可以激活电压门控钙通道增加骨髓干细胞中的Ca2+浓度,上调的Ca2+激活局部黏着斑激酶,增加踝蛋白和黏着斑蛋白表达,促进黏着斑复合体形成。并且,活化的局部黏着斑激酶还可以激活Rho GTP酶,促进F肌动蛋白网络的形成,这些效应协同增强了干细胞体外迁移能力。XIE等[46]发现脉冲电磁场通过诱导位于初级纤毛基部的骨形态发生蛋白受体Ⅱ上调,激活骨形态发生蛋白骨形态发生蛋白Smad1/5/8信号通路,促进大鼠颅骨母细胞的成骨分化和成熟。HE等[47]发现脉冲电磁场通过激活初级纤毛依赖的一氧化氮合酶/一氧化氮/sGC/cGMP/PKG信号通路诱导一氧化氮依赖的成骨分化。脉冲电磁场还可以激活Wnt/β-Catenin信号通路,通过增加脊髓损伤大鼠胫骨组织经典Wnt配体(Wnt1和Wnt10b)的表达,激活下游p-GSK3β和β-Catenin表达,改善脊髓损伤大鼠的骨力学性能[48]。人骨髓间充质干细的Notch信号通路同样可以经脉冲电磁场激活,导致下游基因表达上调,最终增强间充质干细胞的成骨分化[49]。此外,YONG等[50]的研究表明15 Hz/ 1 mT的脉冲电磁场通过蛋白激酶A(protein kinase,PKA)和ERK1/2途径促进大鼠间充质干细胞的成骨分化。以上结果表明脉冲电磁场可以通过Ca2+、骨形态发生蛋白/Smad、一氧化氮/sGC/cGMP/PKG、Wnt/β-Catenin、Notch、PKA/MAPK信号通路促进干细胞的增殖、迁移和成骨分化,见图6。
RANK是破骨细胞分化的关键信号通路,脉冲电磁场抑制RANK通路激活,抑制破骨细胞形成和骨吸收活性。研究表明脉冲电磁场显著抑制RANKL诱导小鼠单核巨噬细胞白血病细胞向破骨细胞的分化,降低抗酒石酸酸性磷酸酶活性和破骨相关基因的表达[51]。白细胞介素1β是骨代谢中的骨吸收相关因子,在诱导骨质流失中起着关键作用,诱导成骨细胞和破骨细胞的RANKL的表达,刺激破骨细胞成熟、增强骨吸收[52]。WANG等[53]的研究结果表明,脉冲电磁场可能通过下调白细胞介素1β表达抑制破骨细胞骨吸收,从而改善卵巢切除小鼠骨结构的恶化。ZHANG等[54]报道称脉冲电磁场抑制细胞内Ca2+振荡和钙调神经磷酸酶活性,抑制RANKL诱导的NFATc1的核转位,从而通过抑制Ca2+-钙调神经磷酸酶-NFATc1信号通路,抑制破骨细胞分化。这些研究提示脉冲电磁场可能通过调节白细胞介素1β和Ca2+代谢抑制RANKL诱导的破骨细胞分化。
血管生成是新骨形成的重要组成部分。脉冲电磁场可通过增强成骨和血管生长之间的相互作用促进骨修复。TEPPER等[55]的研究表明脉冲电磁场的刺激使人脐静脉内皮细胞成纤维细胞生长因子基因增加了150%,成纤维细胞生长因子蛋白增加了5倍,并促进了内皮细胞增殖和成血管化。DELLE MONACHE等[56]用50 Hz/ 1 mT的脉冲电磁场刺激人脐静脉内皮细胞,发现激活了血管内皮生长因子信号转导途径促进内皮细胞增殖和血管形成活性。脉冲电磁场通过成纤维细胞生长因子和血管内皮生长因子信号通路的促血管生成作用为其在骨修复中的治疗功能提供了另一种解释,但仍然需要更多研究来进一步阐明成纤维细胞生长因子和血管内皮生长因子在脉冲电磁场诱导的骨修复中的疗效。
脉冲电磁场刺激启动信号级联反应,以协调的时空方式有效促进成骨和血管生成,促进骨损伤的修复与再生。脉冲电磁场刺激促进骨修复在多种骨骼疾病的治疗中显示出积极的效果,但仍需在动物实验和临床治疗研究中进一步研究,以实现临床转化应用。
2.3.3 磁场与细胞相互作用的机制
磁场效应:磁场影响细胞行为的确切机制尚不完全清楚,机械转导是被广泛接受的假设。细胞膜和细胞骨架感知来自静磁场的机械刺激,激活一系列细胞内信号转导,最终调节基因表达并改变细胞命运。一方面,机械负荷诱导的骨骼变形传递到细胞,导致细胞膜变形,膜蛋白构象的变化[57];另一方面,构成细胞膜基本结构的磷脂双分子层也具有强抗磁各向异性,在磁场的作用下其结构发生重排进而影响膜蛋白的功能[58]。整合素在细胞感知和响应周围环境的机械刺激中起着至关重要的作用,参与从细胞外到细胞内部的机械转导,并最终影响细胞命运[59]。MARYCZ等[60]发现200 mT的静磁场上调了前破骨细胞中整合素β3和整合素α5的基因表达,提高了小鼠胚胎成骨细胞细胞中整合素α3和整合素α5的基因表达。同样地,KASTEN等[61]发现磁场激活的整合素介导的机械应力促进了间充质干细胞的成骨分化。压电蛋白是机械激活的非选择性阳离子通道,也是迄今为止报道的最大质膜离子通道,在骨形成中起关键作用[62]。HAO等[63]观察到接种在磁性纳米支架上的小鼠胚胎成骨细胞接受70-80 mT的磁场刺激后,Piezo1的表达显著上调,碱性磷酸酶活性增强,矿化结节形成增加,成骨分化基因表达上调。虽然目前没有研究显示磁场可以直接激活骨细胞中的压电蛋白通道,但已研究表明磁力可以激活神经母细胞瘤细胞和胚胎肾细胞中的压电通道[64-65],提示磁场直接激活骨细胞的压电蛋白通道的可能机制。磁场诱导的细胞膜结构变化也会影响膜上离子通道的开闭。LEW等[66]发现400 mT的静磁场激活牙髓干细胞钙离子通道,促进钙离子转移到细胞核,激活p38丝裂原活化蛋白激酶信号传导,增加牙髓干细胞的细胞增殖。
膜-细胞骨架相互作用对于整合和传递细胞内和细胞外信号(包括机械转导信号)至关重要。细胞骨架主要包括微管、微丝和中间纤维。微管和微丝在维持细胞形态、调节细胞分裂和细胞迁移方面起着重要作用。磁场能够诱导细胞骨架的重排或结构变化。一方面,细胞骨架与细胞膜相连,细胞膜的变形必然导致细胞骨架的变化;另一方面,微管和微丝都具有很强的抗磁各向异性,因此细胞骨架被认为是磁场与细胞相互作用的重要靶标。人牙髓干细胞接受静磁场刺激后,免疫荧光染色显示细胞骨架变得更致密并重新组装到细胞边缘[67]。转录辅助因子Yes相关蛋白(yes-associated protein,YAP)和具有PDZ结合基序的转录共激活因子(tafazzin,TAZ)对于细胞骨架将机械信号传递到细胞核中至关重要。ZHENG等[67]发现静磁场可以抑制牙髓干细胞YAP/TAZ的磷酸化,增加YAP/TAZ的核定位并促进其下游靶基因的表达。此外,当沉默YAP/TAZ时,静磁场诱导的细胞矿化作用受到明显抑制。骨吸收发生在肌动蛋白环结构形成的闭合区域内,Dong等[39]的研究表明,静磁场显著减少了肌动蛋白环的形成并抑制了骨吸收。
电场效应:脉冲电磁场除了可以产生和静磁场一样的磁场效应,还可以产生电场,调控细胞命运。脉冲电磁场产生的电场引起膜表面电荷的变化,激活细胞膜上的电压门控通道,引起下游级联反应进而调控细胞命运。ZHANG等[45]证明50 Hz/ 1 mT的脉冲电磁场可以激活骨髓干细胞L型电压门控钙通道增加胞内Ca2+浓度,上调的Ca2+激活局部黏着斑激酶和Rho GTPase信号通路增强细胞体外迁移能力。PALL等[43]的研究表明脉冲电磁场可通过Ca2+/一氧化氮/cGMP/PKG途径促进成骨细胞增殖和成熟。细胞膜中还存在其他类型的电压门控离子通道(Na+和K+等),脉冲电磁场可能通过这些离子通道产生生物学效应。ZHENG等[68]使用细胞膜钳技术成功观察到了脉冲电磁场诱导下细胞膜中Na+和K+电流的变化。脉冲电磁场介导的Na+/K+电流变化可能导致细胞代谢、免疫调节、炎症或肿瘤的生理和病理变化,但确切的机制尚不清楚。
综上所述,静磁场和脉冲电磁场均可以通过机械负荷诱导的骨骼变形传递到细胞,导致细胞膜形变,然后引起膜蛋白构象和细胞骨架的变化,最终激活下游一系列信号级联反应,调控细胞命运。此外,脉冲电磁场还可以产生电场,引起细胞膜表面的电荷变化,激活细胞膜上的电压门控通道,调控细胞命运。因此,磁疗在促进骨损伤修复领域具有极大的应用潜力。然而,磁场发挥促进骨修复的生物学功能的机制尚未完全阐明,并且其用于疾病治疗的最佳参数仍不确定,未来还需要进行大量的研究解决这些难题。

2.4 磁性纳米材料联合磁场用于骨损伤修复 鉴于磁性纳米材料和磁场具有促进骨损伤修复的多种生物学效应,越来越多的研究将两者联合应用于骨损伤修复。
2.4.1 触发机械转导作用促进骨损伤修复 细胞感知外部机械刺激并将其转化为生化信号,调控细胞的表型和功能,称为机械转导。机械线索可以刺激成骨分化,增强骨基质和矿物质沉积,并提高骨植入物对骨缺损的修复效果[69]。多项研究证明,磁性纳米复合支架在磁场的作用下发生形变驱动机械转导刺激。BLüMLER [70]研究了在永磁体产生的不均匀磁场中,超顺磁性氧化铁纳米粒子在外部磁场的吸引力和磁性纳米粒子之间的磁偶极的双重作用下驱动磁性纳米支架形变。HAO等[63]进一步使用原子力显微镜观察到磁驱动力作用下的磁性纳米支架的形变,同时支架的形变显著增强了支架上细胞黏附和成骨分化。
整合素是细胞表面重要的黏附分子,介导细胞与细胞外基质的黏附并能感知胞外物理线索,在骨组织细胞感知机械信号中发挥关键作用[71]。MARYCZ等[60]采用改性溶胶-凝胶法制备αFe2O3/γ-Fe2O3纳米复合材料(IOs),IOs和静磁场联用显著上调了小鼠胚胎成骨细胞整合素的表达,激活细胞内的信号网络,促进细胞成骨分化。TANG等[72]在磁性纳米复合涂层联合静磁场用于磁机械刺激促成骨分化的研究中,发现在磁机械刺激下,小鼠胚胎成骨细胞α2β1整合素的表达显著上调,MAPK信号通路中ERK分子的磷酸化增强,表明磁机械刺激诱导整合素介导的MAPK通路激活促进小鼠胚胎成骨细胞成骨分化。YUN等[73]发现磁性纳米复合支架联合静磁场促进成骨细胞分化,成骨分化相关基因水平和碱性磷酸酶活性增加;磁性支架和静磁场联用显著激活了原代小鼠颅骨成骨细胞整合素下游的信号分子,包括局部黏着斑激酶、桩蛋白和Rho A,并观察到p38,ERK和JNK的活化。整合素与转化生长因子β之间存在广泛的串扰,即整合素可以激活潜在的转化生长因子β[74]。HUANG等[75]利用磁性Fe3O4/聚多巴胺涂层支架协同静磁场,增强了人骨髓间充质干细胞体外成骨分化和体内新骨形成。蛋白质组学分析证实了这种促成骨作用与转化生长因子β-Smad信号通路的激活有关。Piezo1是一种机械感受器,赋予成骨细胞机械敏感性,在骨形成中起关键作用[62]。HAO等[63]发现,在磁性纳米复合材料上生长的MC1T3-E3细胞受到静磁场刺激后,Piezo1的表达显著上调,骨形态发生蛋白2基因水平上升,表明骨形态发生蛋白2信号转导是Piezo1在成骨分化中的潜在靶点。这些研究结果表明磁性纳米材料联合磁场刺激的成骨细胞分化可能是整合素-MAPK、转化生长因子β-Smad和Piezo1/骨形态发生蛋白2信号通路激活的结果。
此外,磁机械刺激能诱导巨噬细胞的极化,调节骨损伤免疫微环境用于骨组织的再生修复。HUANG等[76]以胶原纳米纤维为基体,搭载超顺磁性氧化铁纳米粒子,构建了一种能即时控制巨噬细胞极化的超顺磁性水凝胶,在磁场刺激下,磁性纳米支架诱导小鼠单核巨噬细胞白血病细胞由M1型向M2型极化,实现了时空可控的调节组织愈合期炎症微环境,使其与组织再生过程相匹配,最终促进骨愈合。SHAO等[77]证明这种磁机械刺激过触发整合素相关的级联途径,并抑制MAPK通路中JNK的磷酸化,从而诱导M2巨噬细胞极化。M2巨噬细胞通过分泌抗炎细胞因子和生长因子促进骨髓间充质干细胞的体外成骨分化,增强体内颅骨缺损的修复。
2.4.2 增加胞内磁性纳米粒子的含量促进骨损伤修复 磁性会诱导磁性纳米粒子胶体纳米颗粒物理化学性质的变化,并影响其生物学行为与效应。已有研究表明静磁场会抑制细胞外排内吞的超顺磁性氧化铁纳米粒子,导致细胞内超顺磁性氧化铁纳米粒子含量提高近2倍,从而增强间充质干细胞的成骨分化[78]。另一项研究同样发现,磁场刺激下超顺磁性氧化铁纳米粒子-磷酸钙水泥支架上生长的人牙髓干细胞细胞内铁含量显著增加,细胞增殖和成骨活性增强[79]。因此,磁场诱导磁性纳米粒子理化性质的改变会影响其生物学行为,进而影响磁性纳米粒子在骨损伤修复中的生物学效应。
2.4.3 诱导磁性纳米粒子产生更强的磁场促进骨损伤修复 在外加磁场下,超顺磁性氧化铁纳米粒子磁矩排列从混乱变成有序,迅速磁化产生磁场。SHUAI等[80]报道称磁性多孔聚乙醇酸支架内的Fe3O4纳米颗粒在外部静磁场的作用下磁化重排表现出强磁性,Fe3O4纳米颗粒作为内部磁源为生长在支架中的细胞提供直接和更强的磁刺激,激活一系列信号通路,如MAPK、整合素和骨形态发生蛋白2通路,进而显著促进了人脐带衍生间充质干细胞的黏附、增殖和成骨分化。
2.4.4 诱导磁热效应促进骨损伤修复 磁性纳米粒子在交变磁场作用下可产生磁热效应,而温和的热刺激可诱导磁热生物效应,如通过诱导热休克蛋白的上调激活下游信号通路促进骨修复。CAO等[81]报道掺入磁性Fe3O4纳米颗粒的壳聚糖/聚乙二醇水凝胶在磁场作用下产生的热刺激促进了间充质干细胞的成骨分化。TONG等[82]证明热刺激促进干细胞成骨分化与其热休克蛋白47的表达上调有关,因为热休克蛋白47对于成骨相关Ⅰ型胶原蛋白的生物合成和分子成熟是必不可少的。WANG等[83]
进一步证明,磁性纳米粒子在高频磁场下产生的温和热刺激诱导小鼠胚胎成骨细胞和内皮细胞上调热休克蛋白90。热休克蛋白90激活小鼠胚胎成骨细胞中的PI3K/Akt通路,从而显著促进成骨和生物矿化。在内皮细胞中,热休克蛋白90通过稳定p-Akt来促进缺氧诱导因子1α的表达,进而促进新生血管形成。
2.4.5 介导药物靶向和可控释放促进骨损伤修复 磁场远程控制药物靶向和释放用于骨组织创伤修复,具有定位准确、副反应小的优势。GUO等[84]将雌二醇搭载到磁性聚乳酸-羟基乙基共聚物纳米颗粒上,通过外加磁场引导实现骨组织靶向和药物释放,改善卵巢切除诱导的骨质疏松。并且,在脉冲电磁场作用下,支架内的磁性纳米粒子一方面来回振荡加速药物释放,另一方面产生热量改变支架网格结构加速药物释放。FERNANDES等[85]使用低频脉冲电磁场来调节磁性微球中人骨形态发生蛋白的释放曲线防止短时间过高的剂量所带来的副反应。
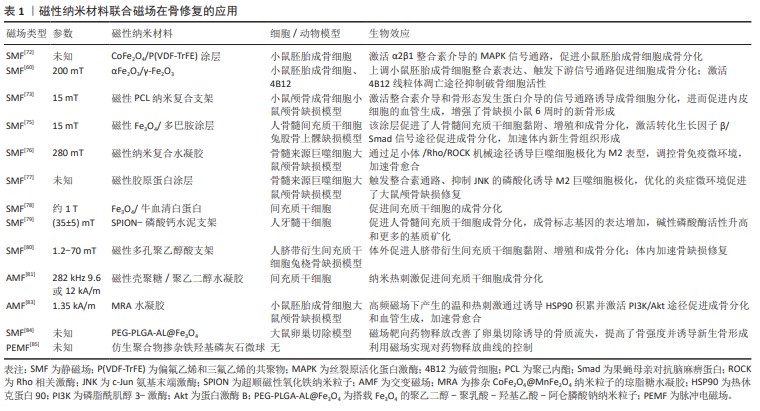
文章总结了联合磁场和磁性纳米材料在骨修复领域的应用,其具有优异的促进损伤骨组织修复的功能,包括促进干细胞的黏附、增殖和成骨分化,内皮细胞成血管化,抑制炎症微环境、诱导巨噬细胞向M2型极化以及靶向释放生物活性分子,加速骨愈合、抑制骨质流失并且无明显的毒副反应,体现了它们在临床实践中的潜在价值,见表1。
2.5 磁场在骨科疾病中的临床应用 目前,磁性纳米粒子用于骨损伤治疗仅限于基础研究,尚缺乏临床试验研究,而磁场已经用于一些骨科疾病的临床治疗,因此,下面简要介绍磁场在临床治疗的应用案例。
2.5.1 骨折 1971年俄罗斯科学家DEGEN和STETSULA首次发现静磁场可以促进骨折愈合[86]。一项对40例20-86岁的手部和手腕骨折患者的临床研究显示,使用插入永磁体的石膏绷带治疗组的平均骨痂形成时间缩短了35%[87]。在2014年发表的一项随机对照试验的荟萃分析表明,与接受安慰剂治疗的患者相比,接受脉冲电磁场治疗的急性骨干骨折患者愈合更快[88]。在2020年的一项研究显示,脉冲电磁场治疗可提高骨折愈合率并减轻患者疼痛,然而现有治疗数据不足以证明脉冲电磁场治疗可缩短骨折愈合时间[89]。因此,未来需要对磁场的最佳频率、振幅和持续时间参数进行更大规模、更高质量的随机对照试验和临床前研究。
2.5.2 骨质疏松症 自1979年FDA批准将脉冲电磁场用于治疗非骨质疏松性骨折以来,脉冲电磁场已被广泛用作治疗原发性骨质疏松症的临床选择。LIU等[90]在中国西南地区开展了一项随机、主动对照的临床试验,研究脉冲电磁场对绝经后骨质疏松症女性的影响,结果表明脉冲电磁场治疗与阿仑膦酸钠治疗绝经后骨质疏松症一样有效,可以改善患者的骨密度、维生素D水平和肌肉力量。另一项对411名受试者的系统评价和荟萃分析表明,脉冲电磁场在预防老年人原发性骨质疏松症骨密度和平衡功能下降方面并不劣于一线治疗(传统药物或运动)[91],但该研究短期随访数据的质量中等,需要高质量的随机对照试验来评估脉冲电磁场治疗原发性骨质疏松症。HUANG等[92]研究表明低频脉冲电磁场可快速有效地缓解原发性骨质疏松症的疼痛,增强骨形成并增加继发性骨质疏松症的骨密度,但脉冲电磁场对原发性骨质疏松症骨密度和骨吸收的影响存在争议。
2.5.3 骨关节炎 一项随机对照试验显示,接受高强度磁性膝关节套治疗的膝关节骨关节炎患者与对照组相比,治疗4 h后疼痛显著降低[93]。HARLOW等[94]发现,当佩戴磁性手环时,髋关节和膝关节骨关节炎患者疼痛减轻。然而,一项随机双盲安慰剂对照交叉试验显示,磁性手环对改善骨关节炎的疼痛、僵硬和身体功能的作用非常微弱[95]。这一矛盾的结果可能归因于磁性手环的磁场强度不足或样本量小,因此,需要进一步进行具有足够样本量的随机对照和双盲试验,以评估静磁场在骨关节炎患者中的有效性。研究发现,与对照组相比,骨关节炎患者接受为期6周的脉冲电磁场治疗后,日常生活质量、疼痛和僵硬均有显著改善[96]。此外,60例膝关节骨关节炎患者接受12 h/d、为期1个月的脉冲电磁场治疗后,疼痛和骨关节炎指数显著降低,疼痛耐受性和身体健康状况也有所改善[97]。然而,OZGUCLU等[98]报道,接受脉冲电磁场治疗2周组与对照组在疼痛、僵硬和躯体机能评分方面无统计学差异。有多种原因可能导致这些相互矛盾的结果:首先,这些研究的临床设计、脉冲电磁场参数和治疗方案各不相同。与应用高频、短时间脉冲电磁场治疗的试验相比,使用低频率和长持续时间的脉冲电磁场治疗在短期内骨关节炎指数评分显著改善;其次,这些研究的样本量太小,可能存在偶然因素的干扰。

| [1] WAWRZYNIAK A, BALAWENDER K. Structural and metabolic changes in bone. Animals (Basel).2022;12(15):1946. [2] SOHN HS, OH JK. Review of bone graft and bone substitutes with an emphasis on fracture surgeries. Biomater Res. 2019;23:9. [3] HO-SHUI-LING A, BOLANDER J, RUSTOM LE, et al. Bone regeneration strategies:engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials. 2018;180:143-162. [4] SUNG B, KIM MH, ABELMANN L. Magnetic microgels and nanogels:physical mechanisms and biomedical applications. Bioeng Transl Med. 2021;6(1):e10190. [5] JIN XH, YANG L, DUAN XJ, et al. In vivo MR imaging tracking of supermagnetic iron-oxide nanoparticle-labeled bone marrow mesenchymal stem cells injected into intra-articular space of knee joints:experiment with rabbit. Zhonghua Yi Xue Za Zhi. 2007;87(45): 3213-3218. [6] KOBAYASHI T, OCHI M, YANADA S, et al. A novel cell delivery system using magnetically labeled mesenchymal stem cells and an external magnetic device for clinical cartilage repair. Arthroscopy. 2008;24(1): 69-76. [7] PARETA RA, TAYLOR E, WEBSTER TJ. Increased osteoblast density in the presence of novel calcium phosphate coated magnetic nanoparticles. Nanotechnology. 2008;19(26):265101. [8] LI M, LIU J, CUI X, et al. Osteogenesis effects of magnetic nanoparticles modified-porous scaffolds for the reconstruction of bone defect after bone tumor resection. Regen Biomater. 2019;6(6):373-381. [9] SADEGHZADEH H, DIANAT-MOGHADAM H, DEL BAKHSHAYESH AR, et al. A review on the effect of nanocomposite scaffolds reinforced with magnetic nanoparticles in osteogenesis and healing of bone injuries. Stem Cell Res Ther. 2023;14(1):194. [10] OBISESAN OS, AJIBOYE TO, MHLANGA SD, et al. Biomedical applications of biodegradable polycaprolactone-functionalized magnetic iron oxides nanoparticles and their polymer nanocomposites. Colloid Surface B. 2023;227:113342. [11] FUKADA E, YASUDA I. On the piezoelectric effect of bone. J Phys Soc Jpn. 1957;12(10):1158-1162. [12] BASSETT CA, PAWLUK RJ, PILLA AA. Augmentation of bone repair by inductively coupled electromagnetic fields. Science. 1974;184(4136): 575-577. [13] BASSETT CA, PAWLUK RJ, BECKER RO. Effects of electric currents on bone in vivo. Nature. 1964;204:652-654. [14] BASSETT CA, PILLA AA, PAWLUK RJ. A non-operative salvage of surgically-resistant pseudarthroses and non-unions by pulsing electromagnetic fields. A preliminary report. Clin Orthop Relat Res. 1977;(124):128-143. [15] RAISZ LG. Physiology and pathophysiology of bone remodeling. Clin Chem. 1999;45(8 Pt 2):1353-1358. [16] GAO L, ZHUANG J, NIE L, et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat Nanotechnol. 2007;2(9):577-583. [17] HUANG DM, HSIAO JK, CHEN YC, et al. The promotion of human mesenchymal stem cell proliferation by superparamagnetic iron oxide nanoparticles. Biomaterials. 2009;30(22):3645-3651. [18] TRAN N, WEBSTER TJ. Increased osteoblast functions in the presence of hydroxyapatite- coated iron oxide nanoparticles. Acta Biomater. 2011;7(3): 1298-1306. [19] XIA Y, GUO Y, YANG ZK, et al. Iron oxide nanoparticle-calcium phosphate cement enhanced the osteogenic activities of stem cells through WNT/β-catenin signaling. Mater Sci Eng C Mater Biol Appl. 2019;104:109955. [20] WANG Q, CHEN B, CAO M, et al. Response of MAPK pathway to iron oxide nanoparticles in vitro treatment promotes osteogenic differentiation of hBMSCs. Biomaterials. 2016;86:11-20. [21] LU JW, YANG F, KE QF, et al. Magnetic nanoparticles modified-porous scaffolds for bone regeneration and photothermal therapy against tumors. Nanomed. 2018;14(3):811-822. [22] LIU L, JIN R, DUAN J, et al. Bioactive iron oxide nanoparticles suppress osteoclastogenesis and ovariectomy-induced bone loss through regulating the TRAF6-p62-CYLD signaling complex. Acta Biomater. 2020;103:281-292. [23] LI MY, FU SX, CAI ZY, et al. Dual regulation of osteoclastogenesis and osteogenesis for osteoporosis therapy by iron oxide hydroxyapatite core/shell nanocomposites. Regen Biomater. 2021;8(5):rbab027. [24] TSAY J, YANG Z, ROSS FP, et al. Bone loss caused by iron overload in a murine model:importance of oxidative stress. Blood. 2010;116(14): 2582-2589. [25] HOU Y, YU LX, XIE WY, et al. Surface roughness and substrate stiffness synergize to drive cellular mechanoresponse. Nano Lett. 2020;20(1): 748-757. [26] BIN S, WANG A, GUO W, et al. Micro magnetic field produced by Fe3O4 nanoparticles in bone scaffold for enhancing cellular activity. Polymers (Basel). 2020;12(9):2045. [27] XIA Y, CHEN H, ZHANG F, et al. Injectable calcium phosphate scaffold with iron oxide nanoparticles to enhance osteogenesis via dental pulp stem cells. Artif Cells Nanomed Biotechnol. 2018;46(sup1):423-433. [28] ZHU Y, JIANG P, LUO B, et al. Dynamic protein corona influences immune-modulating osteogenesis in magnetic nanoparticle (MNP)-infiltrated bone regeneration scaffolds in vivo. Nanoscale. 2019;11(14):6817-6827. [29] ZHU Y, YANG Q, YANG M, et al. Protein corona of magnetic hydroxyapatite scaffold improves cell proliferation via activation of mitogen-activated protein kinase signaling pathway. ACS Nano. 2017;11(4):3690-3704. [30] CHEN H, SUN J, WANG Z, et al. Magnetic cell-scaffold interface constructed by superparamagnetic IONP enhanced osteogenesis of adipose-derived stem cells. ACS Appl Mater Interfaces. 2018;10(51): 44279-44289. [31] ZHANG K, GE W, LUO S, et al. Static magnetic field promotes proliferation, migration, differentiation, and AKT activation of periodontal ligament stem cells. Cells Tissues Organs. 2023;212(4): 317-326. [32] WU H, LI C, MASOOD M, et al. Static magnetic fields regulate T-type calcium ion channels and mediate mesenchymal stem cells proliferation. Cells. 2022;11(15):2460. [33] MAREDZIAK M, TOMASZEWSKI K, POLINCEUSZ P, et al. Static magnetic field enhances the viability and proliferation rate of adipose tissue-derived mesenchymal stem cells potentially through activation of the phosphoinositide 3-kinase/Akt (PI3K/Akt) pathway. Electromagn Biol Med. 2017;36(1):45-54. [34] NA J, ZHANG L, ZHENG L, et al. Static magnetic field regulates proliferation, migration, and differentiation of human dental pulp stem cells by MAPK pathway. Cytotechnology. 2022;74(3):395-405. [35] YANG J, ZHANG J, DING C, et al. Regulation of osteoblast differentiation and iron content in MC3T3-E1 cells by static magnetic field with different intensities. Biol Trace Elem Res. 2018;184(1):214-225. [36] KIM EC, PARK J, NOH G, et al. Effects of moderate intensity static magnetic fields on osteoclastic differentiation in mouse bone marrow cells. Bioelectromagnetics. 2018;39(5):394-404. [37] ZHANG J, DING C, MENG X, et al. Nitric oxide modulates the responses of osteoclast formation to static magnetic fields. Electromagn Biol Med. 2018;37(1):23-34. [38] HOLLIDAY LS, DEAN AD, LIN RH, et al. Low NO concentrations inhibit osteoclast formation in mouse marrow cultures by cGMP-dependent mechanism. Am J Physiol. 1997;272(3 Pt 2):F283-F291. [39] DONG D, YANG J, ZHANG G, et al. 16 T high static magnetic field inhibits receptor activator of nuclear factor kappa-B ligand-induced osteoclast differentiation by regulating iron metabolism in Raw264.7 cells. J Tissue Eng Regen Med. 2019;13(12):2181-2190. [40] WANG X, CHEN B, SUN J, et al. Iron-induced oxidative stress stimulates osteoclast differentiation via NF-kappaB signaling pathway in mouse model. Metabolism. 2018;83:167-176. [41] YAN Z, WANG D, CAI J, et al. High-specificity protection against radiation-induced bone loss by a pulsed electromagnetic field. Sci Adv. 2022;8(34):eabq0222. [42] HAO X, WANG D, YAN Z, et al. Bone deterioration in response to chronichigh-altitude hypoxia is attenuated by a pulsed electromagnetic field via the primary cilium/HIF-1alpha axis. J Bone Miner Res. 2023; 38(4):597-614. [43] PALL ML. Electromagnetic fields act via activation of voltage-gated calcium channels to produce beneficial or adverse effects. J Cell Mol Med. 2013;17(8):958-965. [44] CHEN Y, BRAUN BJ, MENGER MM, et al. Intermittent exposure to a 16 Hz extremely low frequency pulsed electromagnetic field promotes osteogenesis in vitro through activating Piezo 1-induced Ca2+ influx in Osteoprogenitor Cells. J Funct Biomater. 2023;14(3):165. [45] ZHANG Y, YAN J, XU H, et al. Extremely low frequency electromagnetic fields promote mesenchymal stem cell migration by increasing intracellular Ca2+ and activating the FAK/Rho GTPases signaling pathways in vitro. Stem Cell Res Ther. 2018;9(1):143. [46] XIE YF, SHI WG, ZHOU J, et al. Pulsed electromagnetic fields stimulate osteogenic differentiation and maturation of osteoblasts by upregulating the expression of BMPRII localized at the base of primary cilium. Bone. 2016;93:22-32. [47] HE WF, QIN R, GAO YH, et al. The interdependent relationship between the nitric oxide signaling pathway and primary cilia in pulse electromagnetic field-stimulated osteoblastic differentiation. FASEB J. 2022;36(6):e22376. [48] SHAO X, YAN Z, WANG D, et al. Pulsed electromagnetic fields ameliorate skeletal deterioration in bone mass, microarchitecture, and strength by enhancing canonical Wnt signaling-mediated bone formation in rats with spinal cord injury. J Neurotrauma. 2021;38(6):765-776. [49] BAGHERI L, PELLATI A, RIZZO P, et al. Notch pathway is active during osteogenic differentiation of human bone marrow mesenchymal stem cells induced by pulsed electromagnetic fields. J Tissue Eng Regen Med. 2018;12(2):304-315. [50] YONG Y, MING ZD, FENG L, et al. Electromagnetic fields promote osteogenesis of rat mesenchymal stem cells through the PKA and ERK1/2 pathways. J Tissue Eng Regen Med. 2016;10(10): E537-E545. [51] WANG P, LIU J, YANG Y, et al. Differential intensity-dependent effects of pulsed electromagnetic fields on RANKL-induced osteoclast formation, apoptosis, and bone resorbing ability in RAW264.7 cells. Bioelectromagnetics. 2017;38(8):602-612. [52] WEI S, KITAURA H, ZHOU P, et al. IL-1 mediates TNF-induced osteoclastogenesis. J Clin Invest. 2005;115(2):282-290. [530] WANG L, LI Y, XIE S, et al. Effects of pulsed electromagnetic field therapy at different frequencies on bone mass and microarchitecture in osteoporotic mice. Bioelectromagnetics. 2021;42(6):441-454. [54] ZHANG J, XU H, HAN Z, et al. Pulsed electromagnetic field inhibits RANKL-dependent osteoclastic differentiation in RAW264.7 cells through the Ca2+-calcineurin-NFATc1 signaling pathway. Biochem Biophys Res Commun. 2017;482(2):289-295. [55] TEPPER OM, CALLAGHAN MJ, CHANG EI, et al. Electromagnetic fields increase in vitro and in vivo angiogenesis through endothelial release of FGF-2. FASEB J. 2004;18(11):1231-1233. [56] DELLE MONACHE S, ALESSANDRO R, IORIO R, et al. Extremely low frequency electromagnetic fields (ELF-EMFs) induce in vitro angiogenesis process in human endothelial cells. Bioelectromagnetics. 2008;29(8):640-648. [57] PAVALKO FM, NORVELL SM, BURR DB, et al. A model for mechanotransduction in bone cells:the load-bearing mechanosomes. J Cell Biochem. 2003;88(1):104-112. [58] CHIONNA A, DWIKAT M, PANZARINI E, et al. Cell shape and plasma membrane alterations after static magnetic fields exposure. Eur J Histochem. 2003;47(4):299-308. [59] KECHAGIA JZ, IVASKA J, ROCA-CUSACHS P. Integrins as biomechanical sensors of the microenvironment. Nat Rev Mol Cell Biol. 2019;20(8):457-473. [60] MARYCZ K, SOBIERAJSKA P, ROECKEN M, et al. Iron oxides nanoparticles (IOs) exposed to magnetic field promote expression of osteogenic markers in osteoblasts through integrin alpha-3 (INTa-3) activation, inhibits osteoclasts activity and exerts anti-inflammatory action. J Nanobiotechnology. 2020;18(1):33. [61] KASTEN A, MULLER P, BULNHEIM U, et al. Mechanical integrin stress and magnetic forces induce biological responses in mesenchymal stem cells which depend on environmental factors. J Cell Biochem. 2010;111(6):1586-1597. [62] QIN L, HE T, CHEN S, et al. Roles of mechanosensitive channel Piezo1/2 proteins in skeleton and other tissues. Bone Res. 2021;9(1):44. [63] HAO L, LI L, WANG P, et al. Synergistic osteogenesis promoted by magnetically actuated nano-mechanical stimuli. Nanoscale. 2019; 11(48):23423-23437. [64] LEE JU, SHIN W, LIM Y, et al. Non-contact long-range magnetic stimulation of mechanosensitive ion channels in freely moving animals. Nat Mater. 2021;20(7):1029-1036. [65] WU J, GOYAL R, GRANDL J. Localized force application reveals mechanically sensitive domains of Piezo1. Nat Commun. 2016;7:12939. [66] LEW WZ, HUANG YC, HUANG KY, et al. Static magnetic fields enhance dental pulp stem cell proliferation by activating the p38 mitogen-activated protein kinase pathway as its putative mechanism. J Tissue Eng Regen Med. 2018;12(1):19-29. [67] ZHENG L, ZHANG L, CHEN L, et al. Static magnetic field regulates proliferation, migration, differentiation, and YAP/TAZ activation of human dental pulp stem cells. J Tissue Eng Regen Med. 2018;12(10): 2029-2040. [68] ZHENG Y, XIA P, DONG L, et al. Effects of modulation on sodium and potassium channel currents by extremely low frequency electromagnetic fields stimulation on hippocampal CA1 pyramidal cells. Electromagn Biol Med. 2021;40(2):274-285. [69] WANG L, YOU X, ZHANG L, et al. Mechanical regulation of bone remodeling. Bone Res. 2022;10(1):16. [70] BLUMLER P. Magnetic guiding with permanent magnets:Concept, realization and applications to nanoparticles and cells. Cells. 2021; 10(10):2708. [71] SUN Z, GUO SS, FASSLER R. Integrin-mediated mechanotransduction. J Cell Biol. 2016;215(4):445-456. [72] TANG B, SHEN X, YANG Y, et al. Enhanced cellular osteogenic differentiation on CoFe2O4/P(VDF-TrFE) nanocomposite coatings under static magnetic field. Colloids Surf B Biointerfaces. 2021;198:111473. [73] YUN HM, AHN SJ, PARK KR, et al. Magnetic nanocomposite scaffolds combined with static magnetic field in the stimulation of osteoblastic differentiation and bone formation. Biomaterials. 2016;85:88-98. [74] MUNGER JS, SHEPPARD D. Cross talk among TGF-β signaling pathways, integrins, and the extracellular matrix. Cold Spring Harb Perspect Biol. 2011;3(11): a005017. [75] HUANG Z, HE Y, CHANG X, et al. A magnetic iron oxide/polydopamine coating can improve osteogenesis of 3D-printed porous titanium scaffolds with a static magnetic field by upregulating the TGFβ-Smads pathway. Adv Healthc Mater. 2020;9(14):e2000318. [76] HUANG D, XU K, HUANG X, et al. Remotely temporal scheduled macrophage phenotypic transition enables optimized immunomodulatory bone regeneration. Small. 2022;18(39):e2203680. [77] SHAO J, LI J, WENG L, et al. Remote activation of M2 macrophage polarization via magneto-mechanical stimulation to promote osteointegration. ACS Biomater Sci Eng. 2023;9(5):2483-2494. [78] JIANG P, ZHANG Y, ZHU C, et al. Fe3O4/BSA particles induce osteogenic differentiation of mesenchymal stem cells under static magnetic field. Acta Biomater. 2016;46:141-150. [79] XIA Y, CHEN H, ZHAO Y, et al. Novel magnetic calcium phosphate-stem cell construct with magnetic field enhances osteogenic differentiation and bone tissue engineering. Mater Sci Eng C Mater Biol Appl. 2019; 98:30-41. [80] SHUAI CJ, CHENG Y, YANG WJ, et al. Magnetically actuated bone scaffold:Microstructure, cell response and osteogenesis. Composites Part B-Engineering. 2020;192:107986. [81] CAO Z, WANG D, LI Y, et al. Effect of nanoheat stimulation mediated by magnetic nanocomposite hydrogel on the osteogenic differentiation of mesenchymal stem cells. Sci China Life Sci. 2018;61(4):448-456. [82] TONG L, LIAO Q, ZHAO Y, et al. Near-infrared light control of bone regeneration with biodegradable photothermal osteoimplant. Biomaterials. 2019;193:1-11. [83] WANG LT, HU P, JIANG H, et al. Mild hyperthermia-mediated osteogenesis and angiogenesis play a critical role in magnetothermal composite-induced bone regeneration. Nano Today. 2022;43:101401. [84] GUO Y, LIU Y, SHI C, et al. Remote-controllable bone-targeted delivery of estradiol for the treatment of ovariectomy-induced osteoporosis in rats. J Nanobiotechnology. 2021;19(1):248. [85] FERNANDES PATRICIO TM, MUMCUOGLU D, MONTESI M, et al. Bio-inspired polymeric iron-doped hydroxyapatite microspheres as a tunable carrier of rhBMP-2. Mater Sci Eng C Mater Biol Appl. 2021; 119:111410. [86] DEGEN IL, STETSULA VI. Consolidation of bone fragments in a constant magnetic field. Ortop Travmatol Protez. 1971;32(9):45-48. [87] COSTANTINO C, POGLIACOMI F, PASSERA F, et al. Treatment of wrist and hand fractures with natural magnets:preliminary report. Acta Biomed. 2007;78(3):198-203. [88] HANNEMANN PF, MOMMERS EH, SCHOTS JP, et al. The effects of low-intensity pulsed ultrasound and pulsed electromagnetic fields bone growth stimulation in acute fractures: a systematic review and meta-analysis of randomized controlled trials. Arch Orthop Trauma Surg. 2014;134(8):1093-1106. [89] PENG L, FU C, XIONG F, et al. Effectiveness of pulsed electromagnetic fields on bone healing: a systematic review and meta-analysis of randomized controlled trials. Bioelectromagnetics. 2020;41(5):323-337. [90] LIU HF, YANG L, HE HC, et al. Pulsed electromagnetic fields on postmenopausal osteoporosis in Southwest China: a randomized, active-controlled clinical trial. Bioelectromagnetics. 2013;34(4):323-332. [91] ZHU S, LI Y, WANG L, et al. Pulsed electromagnetic fields may be effective for the management of primary osteoporosis: a systematic review and meta-analysis. IEEE Trans Neural Syst Rehabil Eng. 2022; 30:321-328. [92] HUANG LQ, HE HC, HE CQ, et al. Clinical update of pulsed electromagnetic fields on osteoporosis. Chin Med J (Engl). 2008; 121(20):2095-2099. [93] WOLSKO PM, EISENBERG DM, SIMON LS, et al. Double-blind placebo-controlled trial of static magnets for the treatment of osteoarthritis of the knee: results of a pilot study. Altern Ther Health Med. 2004; 10(2):36-43. [94] HARLOW T, GREAVES C, WHITE A, et al. Randomised controlled trial of magnetic bracelets for relieving pain in osteoarthritis of the hip and knee. BMJ. 2004;329(7480):1450-1454. [95] RICHMOND SJ, BROWN SR, CAMPION PD, et al. Therapeutic effects of magnetic and copper bracelets in osteoarthritis: a randomised placebo-controlled crossover trial. Complement Ther Med. 2009;17(5-6):249-256. [96] THAMSBORG G, FLORESCU A, OTURAI P, et al. Treatment of knee osteoarthritis with pulsed electromagnetic fields: a randomized, double-blind, placebo-controlled study. Osteoarthritis Cartilage. 2005; 13(7):575-581. [97] BAGNATO GL, MICELI G, MARINO N, et al. Pulsed electromagnetic fields in knee osteoarthritis: a double blind, placebo-controlled, randomized clinical trial. Rheumatology (Oxf). 2016;55(4):755-762. [98] OZGUCLU E, CETIN A, CETIN M, et al. Additional effect of pulsed electromagnetic field therapy on knee osteoarthritis treatment: a randomized, placebo-controlled study. Clin Rheumatol. 2010;29(8): 927-931. |
| [1] | 俞 磊, 张 巍, 秦 毅, 葛高然, 柏家祥, 耿德春. 贻贝启发接枝骨形态发生蛋白2成骨活性肽的介孔生物玻璃修复股骨髁缺损[J]. 中国组织工程研究, 2025, 29(22): 4629-4638. |
| [2] | 赵 越, 许 燕, 周建平, 张旭婧, 陈宇彤, 靳正阳, 印治涛. 骨组织工程中传统与仿生支架结构设计的差异[J]. 中国组织工程研究, 2025, 29(16): 3458-3468. |
| [3] | 陈家瀚, 奉 超, 黄晓夏, 牛明慧, 王 鑫, 滕 勇. 骨组织工程研究中的二维黑磷材料[J]. 中国组织工程研究, 2025, 29(10): 2124-2131. |
| [4] | 向德剑, 梁晓远, 王胜红, 陈长顺, 田 聪, 闫振兴, 耿 彬, 夏亚一. 微重力导致骨质疏松的机制[J]. 中国组织工程研究, 2025, 29(10): 2132-2140. |
| [5] | 吴尧昆, 刘成林, 付佳豪, 宋 伟, 陈 浩, 席洪钟, 刘 锌, 杜 斌, 孙光权. 中药有效成分结合骨组织工程材料用于骨修复[J]. 中国组织工程研究, 2025, 29(10): 2141-2150. |
| [6] | 刘云翔, 张晓玉, 李 昊, 张 荣, 李利平, 陈崇伟. 金属有机框架材料在骨组织工程和骨科疾病治疗中的多重应用[J]. 中国组织工程研究, 2025, 29(10): 2151-2161. |
| [7] | 伍志鑫, 蒋雯雯, 詹健辉, 李杨书润, 任文燕, 王一宇. 水凝胶:口腔颌面部组织缺损修复中的作用与问题[J]. 中国组织工程研究, 2025, 29(10): 2178-2188. |
| [8] | 李啸群, 徐凯航, 纪 方. 补骨脂异黄酮抑制破骨细胞分化缓解小鼠去卵巢骨质疏松[J]. 中国组织工程研究, 2021, 25(2): 186-190. |
骨损伤是骨骼系统最常见的疾病之一,尽管骨有较强的再生修复能力,但在严重骨折或临界骨缺损(通常> 2 cm,取决于解剖部位)的情况下,骨组织难以实现自身愈合。目前,骨移植是临床上严重骨缺损以及骨不愈合的主要治疗手段,包括自体骨移植、同种异体骨移植和异种骨移植等[2]。其中,自体骨移植是骨缺损治疗的金标准,但存在来源有限、供体部位愈合并发症的缺陷。同种异体和异种骨移植物可避免上述缺陷,但免疫排斥、疾病传播、生物活性丧失等不足限制了其临床应用。
基于组织工程的骨移植替代物有望克服临床骨移植物存在的缺陷,是当前骨缺损修复研究的主要方向。骨组织工程致力于利用生物材料支架、种子细胞和生物活性分子构建仿天然骨骼的骨移植替代物,通过促进细胞迁移、增殖和分化实现骨组织的结构和功能修复[3]。磁性纳米粒子(magnetic nanoparticles),如超顺磁性氧化铁纳米粒子(superparamagnetic iron oxide nanoparticles,SPIONs),具有优异的生物相容性,能在磁场下实现精确的时空可控性而广泛应用于生物医学中,例如磁共振成像、组织工程、磁场药物靶向和基因治疗等[4]。在骨损伤修复领域,磁性纳米粒子早期主要用于标记干细胞,实现干细胞在体内的实时追踪、或是经磁场引导干细胞向骨损伤部位的迁移[5-6]。
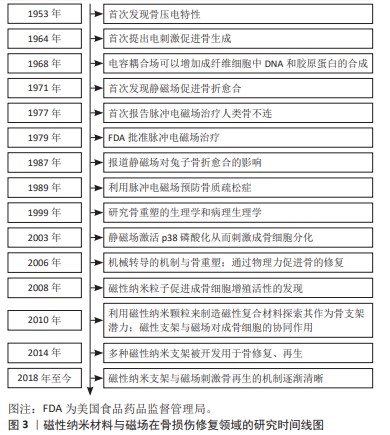
随着磁性纳米粒子促进细胞成骨细胞增殖的生物学功能的发现,磁性纳米粒子用于骨损伤修复的生物学活性和机制研究获得广泛关注[7]。目前,许多研究利用磁性纳米材料构建骨组织工程的骨移植替代物[8]。磁性纳米粒子具有促进成骨细胞分化、抑制破骨细胞的形成和调节免疫微环境等生物效应,还可以调节组织工程支架机械性能、表面形貌等理化性质,并赋予其磁性,促进干细胞的黏附、增殖与分化,促进损伤骨组织的修复与功能重建[9-10]。在骨损伤修复中,除生物刺激外,骨组织还受到外源和内源的物理刺激,其中,基于骨压电特性的发现[11],利用外源磁场产生电磁刺激调节骨细胞生物学行为的策略被广泛用于骨损伤修复。低频和低强度的电磁场可以显著加速骨折愈合[12],1979年美国食品药品监督管理局批准脉冲电磁场用于骨折不愈合治疗。越来越多的证据表明,磁场可显著促进骨骼修复和再生。
近年来,联合磁场与磁性纳米材料的骨组织工程策略,通过机械转导等方式调节多种生物效应促进骨再生修复,逐渐成为研究热点,为骨组织工程的研究提供了新的思路。在此,文章综述了磁性纳米材料与磁场用于骨损伤修复的作用机制,以及联合磁性纳米材料与磁场用于骨损伤修复的研究进展。
1.1.1 检索人及检索时间 第一作者在2023年10月进行检索。
1.1.2 检索文献时限 2003-2023年。
1.1.3 检索数据库 PubMed和Web of Science数据库。
1.1.4 检索词 检索词为“magnetic nanomaterials,magnetic field,bone repair,bone tissue engineering,stem cell,osteoblast,osteoclast”。
1.1.5 检索文献类型 研究原著与综述。
1.1.6 手工检索情况 无。
1.1.7 检索策略 以PubMed数据库检索策略为例,见图1。
1.1.8 检索文献量 初检得到439篇文献,其中PubMed数据库304篇,Web of Science数据库135篇。
1.2 入组标准
1.2.1 纳入标准 磁性纳米材料或磁场治疗骨损伤修复或调控骨再生相关细胞的研究。
1.2.2 排除标准 研究内容与此综述主题相关性差的文献,重复性研究的文献。
1.3 文献质量评估及数据的提取 初步检索到439篇相关文献,按入选标准进行筛选,阅读文献标题、摘要及前言等内容,排除与该综述主题不相关或重复的文献,最终纳入98篇英文文献进行综述,见图2。
首先,虽然磁性纳米材料和磁场在骨损伤修复领域的应用已取得初步成功,但磁性纳米材料、磁场和细胞之间的相互作用机制尚不完全明确。且磁场调控细胞内分子事件的关键参数,包括磁场类型、强度、频率、作用时间和作用方式,以及特定磁场对骨细胞的精确生物效应和潜在机制仍有待明确,未来需进一步阐明其作用机制。其次,目前有关研究主要集中磁性纳米材料和磁场对细胞成骨分化的影响,缺乏对损伤微环境中破骨细胞形成、血管化、免疫调节和神经再生等的研究。最后,尽管到目前为止,磁性支架的体内研究尚未显示出严重的毒性和不良反应,但仍需要长时间和系统研究,以进一步确定其生物安全性。
3.2 作者综述区别于他人他篇的特点 既往相关主题的综述多关注于磁性纳米材料和磁场在骨损伤修复的应用策略,缺乏对磁性纳米材料和磁场用于骨损伤修复机制的全面论述。文章围绕磁性纳米材料和磁场促进骨损伤修复的作用机制进行了相对全面的总结,并综述了磁性纳米材料联合磁场用于骨损伤修复的研究策略,以及磁场在骨损伤相关疾病中的临床应用,为未来相关研究提供了新思路。
3.3 综述的局限性 文章对近年来磁性纳米材料和磁场在骨再生领域的作用及可能存在的机制进行综述。但磁性纳米材料、磁场和细胞之间的相互作用是一个十分复杂的过程,仍然有许多机制尚不明确。文章对现有的研究进行综述,只能反映目前该领域的研究状态,并且某些作用机制尚未完全探明,有待进一步深入的研究。
3.4 综述的重要意义 文章总结了磁性纳米材料和磁场在骨损伤治疗中的相关研究,对其促进骨修复的作用机制和应用策略作了详细综述,提出了基于此的治疗方式用于临床所面临的挑战,为开发用于骨再生领域的新型磁性纳米材料、优化磁性策略提供了理论参考。
3.5 课题组专家的意见和建议 磁性纳米材料的独特性能加速了其在医学上的应用,特别是在磁响应方面。一方面,磁性纳米材料在磁场的协同作用下,通过机械转导效应和磁热效应发挥促新骨形成、血管生成和抑制炎症等功能加速损伤骨组织愈合。另一方面,利用磁性纳米材料和磁场技术,开发能够精准传递药物至骨损伤部位的靶向药物传递系统,这有助于提高治疗效果并降低副反应。
3.5.1 未来的研究方向 ①优化材料设计:进一步研究磁性纳米材料的物理化学性质,以提高其在骨组织中的生物相容性和稳定性。同时,探索新型的磁性纳米材料,以满足不同类型骨损伤修复的需求。可以结合3D打印技术构建损伤部位适配的个性化磁性骨移植物。②探索磁场治疗参数:目前的研究来看,中等强度(1-1 000 mT)的静磁场和低频(0-75 Hz)低强度(0-1 mT)的脉冲电磁场具有促进骨再生的能力。然而关于磁场研究的主要障碍是难以确定某种特定骨病的最佳波形和参数方案。需要深入研究磁场对骨细胞增殖分化和骨组织再生的影响机制,探索最佳的磁场强度、频率和作用时间,以提高治疗效果。③联合治疗:结合其他治疗方法,如药物治疗、物理治疗等,研究磁性纳米材料和磁场在联合治疗中的协同作用,以提高骨损伤修复的整体效果。④临床应用拓展:将磁性纳米材料和磁场应用于其他类型的骨损伤修复拓展其临床应用范围。
3.5.2 临床试验设计举例 ①试验目标:例如评估磁性纳米材料和磁场对骨损伤修复的疗效或安全性;②试验样本:综合考虑年龄、性别等因素选择适宜的样本;③试验设计:严格遵守随机的原则,将随机抽取的患者随机分为试验组和对照组,分别给予磁性纳米材料和磁场治疗和常规治疗;④评价指标:骨愈合时间、疼痛缓解程度和生活质量等;⑤数据统计与分析:对收集到的数据进行统计和分析,评估磁性纳米材料和磁场的安全性和有效性。
总之,尽管磁纳米材料和磁场在骨修复领域的应用已取得显著的进展,但仍面临着许多挑战和问题。一方面,未来还需继续探索新的制备方法和技术提高磁纳米材料的生物相容性和稳定性,以确保其在人体内的长期使用不会对人体产生负面影响同时满足骨修复过程中对于精准度和耐用性的要求;另一方面,需进一步研究磁性纳米材料和磁场在骨修复中的作用机制,以及优化磁疗的参数,明确实现治疗的临界范围,以实现更有效的骨修复效果。
文题释义:
磁性纳米材料:是指结构尺寸在1-100 nm范围内的磁性材料,以及由它们为基本单元构成的磁性材料。磁场:是指传递实物间磁力作用的场。是一种看不见、摸不着,而又客观存在的特殊物质,对放入其中的磁性材料有磁力作用。
骨损伤是骨骼系统最常见的疾病之一,研究具有骨损伤修复活性的骨组织工程材料与策略有望解决临床骨不愈合难题。磁性纳米材料具有促进干细胞成骨分化和抑制破骨细胞形成等生物活性,并在磁场协同下有效促进损伤骨组织愈合,在骨损伤修复中具有广阔的应用前景。
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||