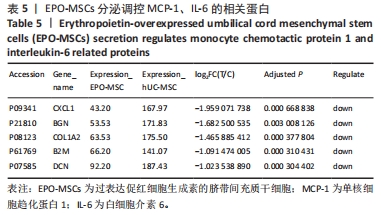
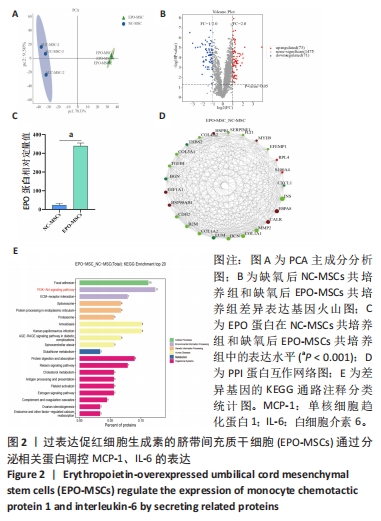
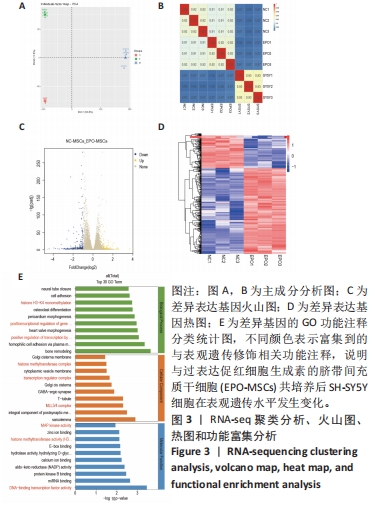
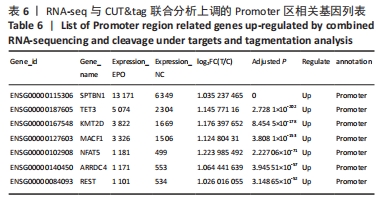
[1] 王文广,王斌,胡颖杰,等.MRI与CT对新生儿缺血缺氧性脑病诊断价值的分析[J].中国妇幼保健,2015,30(4):639-641.
[2] WANG W, JIA L. Regulatory Mechanism of MicroRNA-30b on Neonatal Hypoxic-Ischemic Encephalopathy (HIE). J Stroke Cerebrovasc Dis. 2021;30(3):105553.
[3] LIU CY, AL-WARD H, NGAFFO MEKONTSO F, et al. Experimental Study on the Correlation between miRNA-373 and HIF-1α, MMP-9, and VEGF in the Development of HIE. Biomed Res Int. 2021;2021:5553486.
[4] EDMONDS CJ, HELPS SK, HART D, et al. Minor neurological signs and behavioural function at age 2 years in neonatal hypoxic ischaemic encephalopathy (HIE). Eur J Paediatr Neurol. 2020;27:78-85.
[5] LI JB, WU WS, DU B, et al. Impact of mild hypothermia therapy on hemodynamics during the induction stage in neonates with moderate to severe hypoxic-ischemic encephalopathy. Zhongguo Dang Dai Er Ke Za Zhi. 2021;23(2):133-137.
[6] YE L, FENG Z, DOYCHEVA D, et al. CpG-ODN exerts a neuroprotective effect via the TLR9/pAMPK signaling pathway by activation of autophagy in a neonatal HIE rat model. Exp Neurol. 2018;301(Pt A):70-80.
[7] HU X, LI S, DOYCHEVA DM, et al. Rh-CSF1 Attenuates Oxidative Stress and Neuronal Apoptosis via the CSF1R/PLCG2/PKA/UCP2 Signaling Pathway in a Rat Model of Neonatal HIE. Oxid Med Cell Longev. 2020;2020:6801587.
[8] MASSARO AN, MURTHY K, ZANILETTI I, et al. Short-term outcomes after perinatal hypoxic ischemic encephalopathy: a report from the Children’s Hospitals Neonatal Consortium HIE focus group. J Perinatol. 2015;35(4):290-296.
[9] 余静,薛伟,李亚萍.亚低温联合促红细胞生成素对HIE患儿脑部生理和神经系统发育的改善作用[J].临床研究,2023,31(5):65-68.
[10] LI F, ZHANG K, LIU H, et al. The neuroprotective effect of mesenchymal stem cells is mediated through inhibition of apoptosis in hypoxic ischemic injury. World J Pediatr. 2020;16(2):193-200.
[11] LI T, LIU Y, YU L, et al. Human Umbilical Cord Mesenchymal Stem Cells Protect Against SCA3 by Modulating the Level of 70 kD Heat Shock Protein. Cell Mol Neurobiol. 2018;38(3):641-655.
[12] FRENETTE PS, PINHO S, LUCAS D, et al. Mesenchymal stem cell: keystone of the hematopoietic stem cell niche and a stepping-stone for regenerative medicine. Annu Rev Immunol. 2013;31:285-316.
[13] PARK WS, SUNG SI, AHN SY, et al. Hypothermia augments neuroprotective activity of mesenchymal stem cells for neonatal hypoxic-ischemic encephalopathy. PLoS One. 2015;10(3):e0120893.
[14] PARK M, KIM HM, SHIN HA, et al. Human Pluripotent Stem Cell-Derived Neural Progenitor Cells Promote Retinal Ganglion Cell Survival and Axon Recovery in an Optic Nerve Compression Animal Model. Int J Mol Sci. 2021;22(22):12529.
[15] AHMAD KA, BENNETT MM, JUUL SE, et al. Utilization of Erythropoietin within the United States Neonatal Intensive Care Units from 2008 to 2017. Am J Perinatol. 2021;38(7):734-740.
[16] ZHOU H, HE Y, XIONG W, et al. MSC based gene delivery methods and strategies improve the therapeutic efficacy of neurological diseases. Bioact Mater. 2022;23: 409-437.
[17] BELL KF, BENT RJ, MEESE-TAMURI S, et al. Calmodulin kinase IV-dependent CREB activation is required for neuroprotection via NMDA receptor-PSD95 disruption. J Neurochem. 2013;126(2):274-287.
[18] 班跃耀,孙蕾,马保东,等.促红细胞生成素基因修饰的人脐带间充质干细胞对神经元周期和凋亡的调控作用及机制[J].新乡医学院学报,2023,40(10): 909-916.
[19] HWANG JY, ZUKIN RS. REST, a master transcriptional regulator in neurodegenerative disease. Curr Opin Neurobiol. 2018;48:193-200.
[20] DAY JJ. Genetic and epigenetic editing in
nervous system. Dialogues Clin Neurosci. 2019;21(4):359-368.
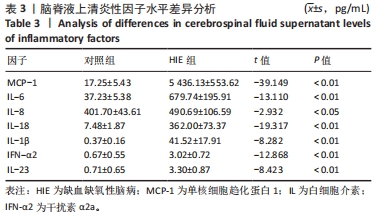
[21] ALY H, KHASHABA MT, EL-AYOUTY M, et al. IL-1beta, IL-6 and TNF-alpha and outcomes of neonatal hypoxic ischemic encephalopathy. Brain Dev. 2006;28(3): 178-182.
[22] 邹良,何秋红,陈秋杨,等. IL-17/IL-23炎症轴在新生儿缺氧缺血性脑病发病中的作用机制探究[J].河北医科大学学报,2023,44(3):305-309.
[23] KUMAR H, KAWAI T, AKIRA S. Pathogen recognition by the innate immune system. Int Rev Immunol. 2011;30(1):16-34.
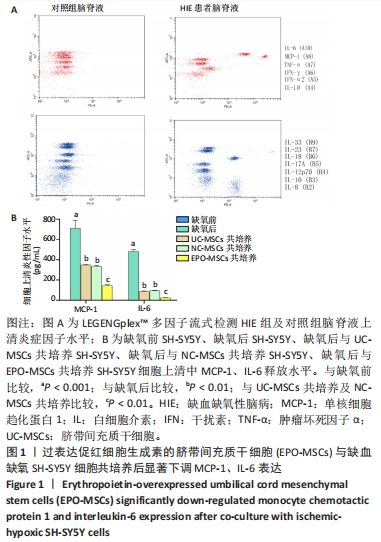
[24] SINGH S, ANSHITA D, RAVICHANDIRAN V. MCP-1: Function, regulation, and involvement in disease. Int Immunopharmacol. 2021;101(Pt B):107598.
[25] 许云鹤,刘永刚,赵小妹,等.血清SAA、RBP4、MCP-1与缺血性脑卒中脑损伤及梗死程度的关系研究[J].临床和实验医学杂志,2018,17(3):255-258.
[26] 张艳华,孔慧霞,张嘉雯,等.血清及脑脊液IL-1β、PCT、MCP-1水平变化在化脓性脑膜炎患儿病情评估及预后判断中的应用价值[J].中国临床实用医学,2017,8(3):45-47.
[27] GROH J, HEINL K, KOHL B, et al. Attenuation of MCP-1/CCL2 expression ameliorates neuropathy in a mouse model for Charcot-Marie-Tooth 1X. Hum Mol Genet. 2010;19(18):3530-3543.
[28] KOLATTUKUDY PE, NIU J. Inflammation, endoplasmic reticulum stress, autophagy, and the monocyte chemoattractant protein-1/CCR2 pathway. Circ Res. 2012; 110(1):174-189.
[29] HUANG XY, HU QP, SHI HY, et al. Everolimus inhibits PI3K/Akt/mTOR and NF-kB/IL-6 signaling and protects seizure-induced brain injury in rats. J Chem Neuroanat. 2021;114:101960.
[30] HOU SM, CHEN PC, LIN CM, et al. CXCL1 contributes to IL-6 expression in osteoarthritis and rheumatoid arthritis synovial fibroblasts by CXCR2, c-Raf, MAPK, and AP-1 pathway. Arthritis Res Ther. 2020;22(1):251.
[31] ZHANG X, ZHANG F, YAO F, et al. Bergenin has neuroprotective effects in mice with ischemic stroke through antioxidative stress and anti-inflammation via regulating Sirt1/FOXO3a/NF-κB signaling. Neuroreport. 2022;33(13):549-560.
[32] ZHONG Q, ZOU Y, LIU H, et al. Toll-like receptor 4 deficiency ameliorates β2-microglobulin induced age-related cognition decline due to neuroinflammation in mice. Mol Brain. 2020;13(1):20.
[33] CHENG Y, WANG G, ZHAO L, et al. Periplocymarin Induced Colorectal Cancer Cells Apoptosis Via Impairing PI3K/AKT Pathway. Front Oncol. 2021;11:753598.
[34] TIAN Q, GUO Y, FENG S, et al. Inhibition of CCR2 attenuates neuroinflammation and neuronal apoptosis after subarachnoid hemorrhage through the PI3K/Akt pathway. J Neuroinflammation. 2022;19(1):312.
[35] NIE Y, SHU C, SUN X. Cooperative binding of transcription factors in the human genome. Genomics. 2020;112(5):3427-3434.
[36] WANG S, MEYER DH, SCHUMACHER B. H3K4me2 regulates the recovery of protein biosynthesis and homeostasis following DNA damage. Nat Struct Mol Biol. 2020;27(12):1165-1177.
[37] SOLLINGER C, LILLIS J, MALIK J, et al. Erythropoietin Signaling Regulates Key Epigenetic and Transcription Networks in Fetal Neural Progenitor Cells. Sci Rep. 2017;7(1):14381.
[38] KAYA-OKUR HS, WU SJ, CODOMO CA, et al. CUT&Tag for efficient epigenomic profiling of small samples and single cells. Nat Commun. 2019;10(1):1930.
[39] RYAN BJ, BENGOA-VERGNIORY N, WILLIAMSON M, et al. REST Protects Dopaminergic Neurons from Mitochondrial and α-Synuclein Oligomer Pathology in an Alpha Synuclein Overexpressing BAC-Transgenic Mouse Model. J Neurosci. 2021;41(16):3731-3746.
[40] LAM XJ, MANIAM S, CHEAH PS, et al. REST in the Road Map of Brain Development. Cell Mol Neurobiol. 2023;43(7):3417-3433.
[41] PERERA A, EISEN D, WAGNER M, et al. TET3 is recruited by REST for context-specific hydroxymethylation and induction of gene expression. Cell Rep. 2015; 11(2):283-294.
[42] ABDERRAHMANI A, STEINMANN M, PLAISANCE V, et al. The transcriptional repressor REST determines the cell-specific expression of the human MAPK8IP1 gene encoding IB1 (JIP-1). Mol Cell Biol. 2001;21(21):7256-7267.
[43] MARTIN D, ALLAGNAT F, GESINA E, et al. Specific silencing of the REST target genes in insulin-secreting cells uncovers their participation in beta cell survival. PLoS One. 2012;7(9):e45844.
[44] KINUGAWA S, WANG Z, KAMINSKI PM, et al. Limited exercise capacity in heterozygous manganese superoxide dismutase gene-knockout mice: roles of superoxide anion and nitric oxide. Circulation. 2005;111(12):1480-1486.
|