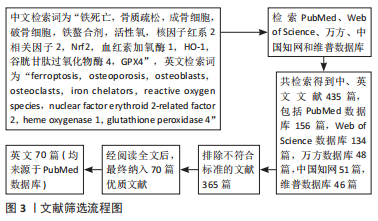
[1] LANE NE. Epidemiology, etiology, and diagnosis of osteoporosis. Am J Obstet Gynecol. 2006;194(2 Suppl):S3-11.
[2] SRIVASTAVA M, DEAL C. Osteoporosis in elderly: prevention and treatment. Clin Geriatr Med. 2002;18(3):529-555.
[3] BACCARO LF, CONDE DM, COSTA-PAIVA L, et al. The epidemiology and management of postmenopausal osteoporosis: a viewpoint from Brazil. Clin Interv Aging. 2015;10:583-591.
[4] WEINSTEIN RS, MANOLAGAS SC. Apoptosis and osteoporosis. Am J Med. 2000;108(2):153-164.
[5] KIM KH, LEE MS. Autophagy--a key player in cellular and body metabolism. Nat Rev Endocrinol. 2014;10(6):322-337.
[6] TIAN Q, WU S, DAI Z, et al. Iron overload induced death of osteoblasts in vitro: involvement of the mitochondrial apoptotic pathway. PeerJ. 2016;4: e2611.
[7] TIAN Q, QIN B, GU Y, et al. ROS-Mediated Necroptosis Is Involved in Iron Overload-Induced Osteoblastic Cell Death. Oxid Med Cell Longev. 2020; 2020:1295382.
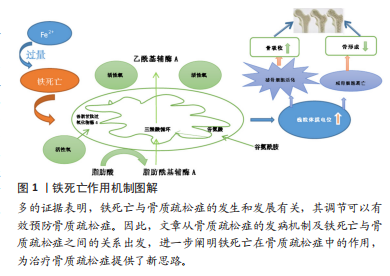
[8] GE W, JIE J, YAO J, et al. Advanced glycation end products promote osteoporosis by inducing ferroptosis in osteoblasts. Mol Med Rep. 2022; 25(4):140.
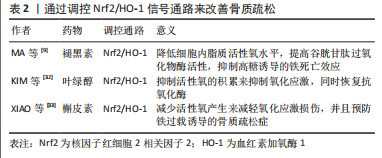
[9] MA H, WANG X, ZHANG W, et al. Melatonin Suppresses Ferroptosis Induced by High Glucose via Activation of the Nrf2/HO-1 Signaling Pathway in Type 2 Diabetic Osteoporosis. Oxid Med Cell Longev. 2020;2020:9067610.
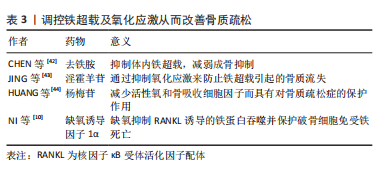
[10] NI S, YUAN Y, QIAN Z, et al. Hypoxia inhibits RANKL-induced ferritinophagy and protects osteoclasts from ferroptosis. Free Radic Biol Med. 2021;169: 271-282.
[11] DING H, CHEN S, PAN X, et al. Transferrin receptor 1 ablation in satellite cells impedes skeletal muscle regeneration through activation of ferroptosis. J Cachexia Sarcopenia Muscle. 2021;12(3):746-768.
[12] LIU P, WANG W, LI Z, et al. Ferroptosis: A New Regulatory Mechanism in Osteoporosis. Oxid Med Cell Longev. 2022;2022:2634431.
[13] DIXON SJ, LEMBERG KM, LAMPRECHT MR, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060-1072.
[14] DIXON SJ, STOCKWELL BR. The role of iron and reactive oxygen species in cell death. Nat Chem Biol. 2014;10(1):9-17.
[15] STOCKWELL BR, FRIEDMANN ANGELI JP, BAYIR H, et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell. 2017;171(2):273-285.
[16] STRASSER A, O’CONNOR L, DIXIT VM. Apoptosis signaling. Annu Rev Biochem. 2000;69:217-245.
[17] WANG X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15(22):2922-2933.
[18] KROEMER G, GALLUZZI L, VANDENABEELE P, et al. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009;16(1):3-11.
[19] VANLANGENAKKER N, VANDEN BERGHE T, VANDENABEELE P. Many stimuli pull the necrotic trigger, an overview. Cell Death Differ. 2012;19(1):75-86.
[20] BERGMANN A. Autophagy and cell death: no longer at odds. Cell. 2007; 131(6):1032-1034.
[21] YANG Y, KLIONSKY DJ. Autophagy and disease: unanswered questions. Cell Death Differ. 2020;27(3):858-871.
[22] LEDESMA-COLUNGA MG, WEIDNER H, VUJIC SPASIC M, et al. Shaping the bone through iron and iron-related proteins. Semin Hematol. 2021; 58(3):188-200.
[23] LIU F, ZHANG WL, MENG HZ, et al. Regulation of DMT1 on autophagy and apoptosis in osteoblast. Int J Med Sci. 2017;14(3):275-283.
[24] MENG HZ, ZHANG WL, LIU F, et al. Advanced Glycation End Products Affect Osteoblast Proliferation and Function by Modulating Autophagy Via the Receptor of Advanced Glycation End Products/Raf Protein/Mitogen-activated Protein Kinase/Extracellular Signal-regulated Kinase Kinase/Extracellular Signal-regulated Kinase (RAGE/Raf/MEK/ERK) Pathway. J Biol Chem. 2015;290(47):28189-28199.
[25] ZHANG WL, MENG HZ, YANG MW. Regulation of DMT1 on Bone Microstructure in Type 2 Diabetes. Int J Med Sci. 2015;12(5):441-449.
[26] WANG X, MA H, SUN J, et al. Mitochondrial Ferritin Deficiency Promotes Osteoblastic Ferroptosis Via Mitophagy in Type 2 Diabetic Osteoporosis. Biol Trace Elem Res. 2022;200(1):298-307.
[27] ZHANG DD. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab Rev. 2006;38(4):769-789.
[28] DODSON M, CASTRO-PORTUGUEZ R, ZHANG DD. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019;23:101107.
[29] SONG X, LONG D. Nrf2 and Ferroptosis: A New Research Direction for Neurodegenerative Diseases. Front Neurosci. 2020;14:267.
[30] XU Y, SANG W, ZHONG Y, et al. CoCrMo-Nanoparticles induced peri-implant osteolysis by promoting osteoblast ferroptosis via regulating Nrf2-ARE signalling pathway. Cell Prolif. 2021;54(12):e13142.
[31] LEVI S, CORSI B, BOSISIO M, et al. A human mitochondrial ferritin encoded by an intronless gene. J Biol Chem. 2001;276(27):24437-24440.
[32] KIM EN, TRANG NM, KANG H, et al. Phytol Suppresses Osteoclast Differentiation and Oxidative Stress through Nrf2/HO-1 Regulation in RANKL-Induced RAW264.7 Cells. Cells. 2022;11(22):3596.
[33] XIAO J, ZHANG G, CHEN B, et al. Quercetin protects against iron overload-induced osteoporosis through activating the Nrf2/HO-1 pathway. Life Sci. 2023;322:121326.
[34] QIN Y, QIAO Y, WANG D, et al. Ferritinophagy and ferroptosis in cardiovascular disease: Mechanisms and potential applications. Biomed Pharmacother. 2021;141:111872.
[35] ARNETT TR, ORRISS IR. Metabolic properties of the osteoclast. Bone. 2018; 115:25-30.
[36] ISHII KA, FUMOTO T, IWAI K, et al. Coordination of PGC-1beta and iron uptake in mitochondrial biogenesis and osteoclast activation. Nat Med. 2009;15(3):259-266.
[37] ZHANG J. The osteoprotective effects of artemisinin compounds and the possible mechanisms associated with intracellular iron: A review of in vivo and in vitro studies. Environ Toxicol Pharmacol. 2020;76:103358.
[38] YANG Y, LIN Y, WANG M, et al. Targeting ferroptosis suppresses osteocyte glucolipotoxicity and alleviates diabetic osteoporosis. Bone Res. 2022;10(1):26.
[39] YANG J, DONG D, LUO X, et al. Iron Overload-Induced Osteocyte Apoptosis Stimulates Osteoclast Differentiation Through Increasing Osteocytic RANKL Production In Vitro. Calcif Tissue Int. 2020;107(5):499-509.
[40] YANG RZ, XU WN, ZHENG HL, et al. Involvement of oxidative stress-induced annulus fibrosus cell and nucleus pulposus cell ferroptosis in intervertebral disc degeneration pathogenesis. J Cell Physiol. 2021;236(4):2725-2739.
[41] LU S, SONG Y, LUO R, et al. Ferroportin-Dependent Iron Homeostasis Protects against Oxidative Stress-Induced Nucleus Pulposus Cell Ferroptosis and Ameliorates Intervertebral Disc Degeneration In Vivo. Oxid Med Cell Longev. 2021;2021:6670497.
[42] CHEN B, YAN YL, LIU C, et al. Therapeutic effect of deferoxamine on iron overload-induced inhibition of osteogenesis in a zebrafish model. Calcif Tissue Int. 2014;94(3):353-360.
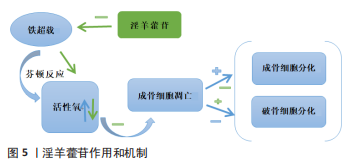
[43] JING X, DU T, CHEN K, et al. Icariin protects against iron overload-induced bone loss via suppressing oxidative stress. J Cell Physiol. 2019;234(7):10123-10137.
[44] HUANG Q, GAO B, WANG L, et al. Protective effects of myricitrin against osteoporosis via reducing reactive oxygen species and bone-resorbing cytokines. Toxicol Appl Pharmacol. 2014;280(3):550-560.
[45] WU D, WEN X, LIU W, et al. Comparison of the effects of deferasirox, deferoxamine, and combination of deferasirox and deferoxamine on an aplastic anemia mouse model complicated with iron overload. Drug Des Devel Ther. 2018;12:1081-1091.
[46] WAN C, GILBERT SR, WANG Y, et al. Activation of the hypoxia-inducible factor-1alpha pathway accelerates bone regeneration. Proc Natl Acad Sci U S A. 2008;105(2):686-691.
[47] ZHAO Q, SHEN X, ZHANG W, et al. Mice with increased angiogenesis and osteogenesis due to conditional activation of HIF pathway in osteoblasts are protected from ovariectomy induced bone loss. Bone. 2012;50(3):763-770.
[48] CHE J, YANG J, ZHAO B, et al. The Effect of Abnormal Iron Metabolism on Osteoporosis. Biol Trace Elem Res. 2020;195(2):353-365.
[49] CHUNG JH, KIM YS, NOH K, et al. Deferoxamine promotes osteoblastic differentiation in human periodontal ligament cells via the nuclear factor erythroid 2-related factor-mediated antioxidant signaling pathway. J Periodontal Res. 2014;49(5):563-573.
[50] XU Z, SUN W, LI Y, et al. The regulation of iron metabolism by hepcidin contributes to unloading-induced bone loss. Bone. 2017;94:152-161.
[51] ZHANG J, ZHENG L, WANG Z, et al. Lowering iron level protects against bone loss in focally irradiated and contralateral femurs through distinct mechanisms. Bone. 2019;120:50-60.
[52] DOWDLE WE, NYFELER B, NAGEL J, et al. Selective VPS34 inhibitor blocks autophagy and uncovers a role for NCOA4 in ferritin degradation and iron homeostasis in vivo. Nat Cell Biol. 2014;16(11):1069-1079.
[53] ALVAREZ SW, SVIDERSKIY VO, TERZI EM, et al. NFS1 undergoes positive selection in lung tumours and protects cells from ferroptosis. Nature. 2017; 551(7682):639-643.
[54] LEMMA S, SBOARINA M, PORPORATO PE, et al. Energy metabolism in osteoclast formation and activity. Int J Biochem Cell Biol. 2016;79:168-180.
[55] LI B, LEE WC, SONG C, et al. Both aerobic glycolysis and mitochondrial respiration are required for osteoclast differentiation. FASEB J. 2020;34(8): 11058-11067.
[56] WORTZEL I, DROR S, KENIFIC CM, et al. Exosome-Mediated Metastasis: Communication from a Distance. Dev Cell. 2019;49(3):347-360.
[57] LU J, YANG J, ZHENG Y, et al. Extracellular vesicles from endothelial progenitor cells prevent steroid-induced osteoporosis by suppressing the ferroptotic pathway in mouse osteoblasts based on bioinformatics evidence. Sci Rep. 2019;9(1):16130.
[58] YANG RZ, XU WN, ZHENG HL, et al. Exosomes derived from vascular endothelial cells antagonize glucocorticoid-induced osteoporosis by inhibiting ferritinophagy with resultant limited ferroptosis of osteoblasts. J Cell Physiol. 2021;236(9):6691-6705.
[59] FINCK H, HART AR, JENNINGS A, et al. Is there a role for vitamin C in preventing osteoporosis and fractures? A review of the potential underlying mechanisms and current epidemiological evidence. Nutr Res Rev. 2014; 27(2):268-283.
[60] DEYHIM F, STRONG K, DEYHIM N, et al. Vitamin C reverses bone loss in an osteopenic rat model of osteoporosis. Int J Vitam Nutr Res. 2018;88(1-2): 58-64.
[61] SZARKA A, KAPUY O, LŐRINCZ T, et al. Vitamin C and Cell Death. Antioxid Redox Signal. 2021;34(11):831-844.
[62] SHEN CL, CHYU MC, WANG JS. Tea and bone health: steps forward in translational nutrition. Am J Clin Nutr. 2013;98(6 Suppl):1694S-1699S.
[63] CHEN K, QIU P, YUAN Y, et al. Pseurotin A Inhibits Osteoclastogenesis and Prevents Ovariectomized-Induced Bone Loss by Suppressing Reactive Oxygen Species. Theranostics. 2019;9(6):1634-1650.
[64] HONG G, CHEN Z, HAN X, et al. A novel RANKL-targeted flavonoid glycoside prevents osteoporosis through inhibiting NFATc1 and reactive oxygen species. Clin Transl Med. 2021;11(5):e392.
[65] BERSUKER K, HENDRICKS JM, LI Z, et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575(7784):688-692.
[66] INGOLD I, BERNDT C, SCHMITT S, et al. Selenium Utilization by GPX4 Is Required to Prevent Hydroperoxide-Induced Ferroptosis. Cell. 2018;172(3): 409-422.e21.
[67] CONRAD M, PRONETH B. Selenium: Tracing Another Essential Element of Ferroptotic Cell Death. Cell Chem Biol. 2020;27(4):409-419.
[68] ALIM I, CAULFIELD JT, CHEN Y, et al. Selenium Drives a Transcriptional Adaptive Program to Block Ferroptosis and Treat Stroke. Cell. 2019;177(5): 1262-1279.e25.
[69] DOUGALL WC. Molecular pathways: osteoclast-dependent and osteoclast-independent roles of the RANKL/RANK/OPG pathway in tumorigenesis and metastasis. Clin Cancer Res. 2012;18(2):326-335.
[70] BAEK JM, KIM JY, YOON KH, et al. Ebselen Is a Potential Anti-Osteoporosis Agent by Suppressing Receptor Activator of Nuclear Factor Kappa-B Ligand-Induced Osteoclast Differentiation In vitro and Lipopolysaccharide-Induced Inflammatory Bone Destruction In vivo. Int J Biol Sci. 2016;12(5):478-488. |