中国组织工程研究 ›› 2024, Vol. 28 ›› Issue (4): 574-580.doi: 10.12307/2023.896
• 组织构建综述 tissue construction review • 上一篇 下一篇
哺乳动物雷帕霉素靶蛋白与运动、高脂肪/高盐饮食和衰老的关系
王士杰,文登台,王京峰,高颖晖
- 鲁东大学体育学院,山东省烟台市 264025
-
收稿日期:2022-12-26接受日期:2023-01-19出版日期:2024-02-08发布日期:2023-07-14 -
通讯作者:文登台,博士,副教授,硕士研究生导师,鲁东大学体育学院,山东省烟台市 264025 -
作者简介:王士杰,男,1997年生,山东省烟台市人,汉族,鲁东大学在读硕士研究生,主要从事体育教育训练学研究。 -
基金资助:国家自然科学基金(32000832),项目负责人:文登台;山东省自然科学基金(ZR2020QC096),项目负责人:文登台
Mammalian target of rapamycin in relation to exercise, high fat/high salt diet, and aging
Wang Shijie, Wen Dengtai, Wang Jingfeng, Gao Yinghui
- School of Physical Education, Ludong University, Yantai 264025, Shandong Province, China
-
Received:2022-12-26Accepted:2023-01-19Online:2024-02-08Published:2023-07-14 -
Contact:Wen Dengtai, MD, Associate professor, Master’s supervisor, School of Physical Education, Ludong University, Yantai 264025, Shandong Province, China -
About author:Wang Shijie, Master candidate, School of Physical Education, Ludong University, Yantai 264025, Shandong Province, China -
Supported by:National Natural Science Foundation of China, No. 32000832 (to WDT); Natural Science Foundation of Shandong Province, No. ZR2020QC096 (to WDT)
摘要:
文题释义:
哺乳动物雷帕霉素靶蛋白(mammalian target of rapamycin,mTor):mTor复合物是细胞生长和代谢的中央调节剂,它整合了来自氨基酸、营养素和细胞外信号的输入,通过对这些信息进行整合加工mTor可以进一步调节下游信息最终实现生理效应,如影响细胞生长和器官发育。mTor与Tor同源,通过对诸多文献进行分析,发现包含酵母在内的多个非哺乳动物的真核生物雷帕霉素靶蛋白(target of rapamycin,Tor)也可以统称为mTor。mTor复合物(mTorC1、mTorC2):两种蛋白质复合物 mTorC1和mTorC2均包含mTor,这两种蛋白质复合物从酵母到人类在进化上是保守的。mTorC1是临床上重要的药物雷帕霉素的直接靶点,雷帕霉素变构抑制 mTorC1,而它仅间接抑制 mTorC2。mTorC1 和 mTorC2 的活性对于维持代谢稳态、预防疾病和延长健康寿命是必要的。
背景:衰老是一个不可逆的过程,其特征与基因、饮食和环境有关。哺乳动物雷帕霉素靶蛋白(mammalian target of rapamycin,mTor)作为生长发育的中心调节剂对衰老、运动及不良饮食导致的负面影响具有调节作用,这些作用与mTor及其复合物的活性相互关联。然而各因素间的相互联系仍不十分清楚,如mTor、运动对衰老的影响。
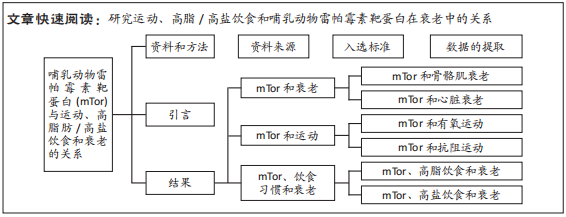
目的:拟通过研究运动、高脂/高盐饮食和mTor在衰老中的关系,从而对衰老的防治机制有更加全面的认识。方法:①文献资料法,通过在CNKI及Web of Science核心合集数据库中进行检索,围绕“mTor基因(mammalian target of rapamycin/mTor)、运动(exercise)、高脂肪/高盐饮食(high-fat diet/high-salt diet)及衰老(aging)” 等关键词进行关键词的组合搜索,对相关文献进行检索、查阅和筛选,为文章提供理论支撑。②对比分析法,通过对所得到的有效文献进行仔细阅读,比较各文献间的差异,为文章论点提供理论基础。③通过对比分析文献间的异同点,明确各指标的定义及关系,从而理清文章思路。
结果与结论:mTor与衰老密切相关,通过分析文献,认为mTor的2个复合物mTorC1和mTorC2在衰老、运动和骨骼肌的生长发育中起着重要作用。此外,mTor介导的S6K1、Akt、FOXO和4E-BP1信号通路与运动、高脂饮食、高盐饮食和骨骼肌/心脏衰老密切相关。
https://orcid.org/0000-0002-8969-9027(王士杰)
中国组织工程研究杂志出版内容重点:组织构建;骨细胞;软骨细胞;细胞培养;成纤维细胞;血管内皮细胞;骨质疏松;组织工程
中图分类号:
引用本文
王士杰, 文登台, 王京峰, 高颖晖. 哺乳动物雷帕霉素靶蛋白与运动、高脂肪/高盐饮食和衰老的关系[J]. 中国组织工程研究, 2024, 28(4): 574-580.
Wang Shijie, Wen Dengtai, Wang Jingfeng, Gao Yinghui. Mammalian target of rapamycin in relation to exercise, high fat/high salt diet, and aging[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 574-580.
60岁以后,身体的许多器官和组织发生了很大的变化,被称为人类老化综合征,在此阶段人体抵抗力及身体内部各器官间的协调性下降[1]。mTor信号与多种生物体的衰老过程密切相关,在几种类型的人类年龄相关疾病,如癌症、糖尿病、心血管疾病和神经退行性疾病中,已经观察到mTor活性失调,并与正常衰老过程密切相关[2]。mTorC1是一种中央调控的激酶,可以调节细胞的生长和蛋白质的合成,抑制mTorC1可能会减少细胞蛋白质的产生,而mTorC1的激活则可能会引起衰老导致的有害积累[3]。对果蝇的研究表明,减少mTor或Raptor同系物的表达会延长果蝇的寿命[4]。这种灭活mTorC1的方法在小鼠身上也得到了证实,直接敲除mTorC1可使小鼠的寿命延长20%,与年龄有关的病理现象也明显减少[5]。在黑腹果蝇中,通过操纵负责激活TorC1上游营养感应通路的基因,下调TorC1的活性,可使寿命延长约15%[6]。
mTor可以对许多不同的环境和内分泌刺激产生敏感反应。作为生长和增殖的中心调节器,mTor也在调节新陈代谢和衰老方面起作用[7]。mTorC1在衰老中起着重要的调节作用,可以调节衰老的许多方面,而雷帕霉素可以防止过度衰老,改善体外功能[8]。首先,在抑制mTorC1过程中,mRNA翻译普遍减少,通过减少蛋白质毒性和氧化应激的积累来延缓衰老[9];其次,mTorC1信号可以间接增强肠道干细胞的功能,在热量限制期间延长寿命[10];最后,真核生物雷帕霉素靶蛋白(target of rapamycin,Tor)可能参与自噬相关蛋白1抗体(ATG1/ULK1)的磷酸化过程,也可能在控制神经元功能方面起作用,在人类的生长和发育中起着重要作用[11]。然而,对于生长发育基本结束的成年人来说,Tor主要影响衰老的调节过程和与营养有关的生理方面[12]。抑制mTorC1可以通过刺激自噬来延缓衰老,自噬可以清除老化和功能失调的线粒体,这些线粒体的积累与衰老和年龄相关的疾病有关[2]。然而,最近的研究表明,抑制mTorC1可能没有抗衰老的功能,但它可能对与年龄有关的疾病有延迟作用[13-14]。
虽然mTor复合物2(mTor complex2,mTorC2)和mTorC1有相反的协调作用,但它们是相互关联的。mTorC1下游的核糖体S6激酶1(ribosomal S6 kinase 1,S6K1)直磷接酸化mTorC2的核心成分rictor (rapamycin-insensitive companion of mTOR),并促进mTorC2依赖的磷酸化蛋白激酶B(Akt-Ser473,PKB)磷酸化的负调控作用[14]。mTorc2激活的苏氨酸激酶(protein kinase,Akt)通过磷酸化mTorC1抑制因子(tuberous sclerosis complex,TSC1-2)来增强mTorC1的活性[15],抑制mTorC2对哺乳动物的寿命和健康的负面影响[16]。与此同时,研究表明Tor可能通过影响胰岛素信号来参与衰老[17]。例如,在果蝇中,Tor功能的降低会导致胰岛素水平的增加。对营养物质敏感的TSC1-2/Tor通路与胰岛素受体通路在控制代谢和衰老方面有多种互动方式[18]。因此,当Tor复合体,即TorC1被抑制时,它可以通过mRNA翻译延迟衰老,增强干细胞功能,并刺激自噬,从而延长寿命。相反,另一个Tor复合体TorC2被抑制时,对生物体的寿命和健康有负面影响,而且Tor也可能通过影响胰岛素信号参与衰老。
此外,自噬也参与了人体的衰老过程。自噬是一种溶酶体降解通路,在清除蛋白质聚集物和受损或多余的细胞器(包括线粒体)以维持细胞平衡方面起着关键作用[19]。自噬可以保护细胞免受各种与年龄有关的负面影响,包括缺氧、内质网(endoplasmic reticulum,ER)压力和氧化压力,氨基酸传感器(amino acid sensing,AAs)通过mTORC1活性调节自噬[19]。据报道,支链氨基酸亮氨酸(branched chain amino acid leucine,BCAAs)激活肝脏mTOR,可抑制脂质诱导的肝脏自噬,并引起肝脏脂质中毒[20]。研究表明,低蛋白膳食通过抑制mTorC1诱导的2型糖尿病激活肥胖大鼠的自噬而发挥肾脏保护作用[21]。由此可知,mTor联合热量限制及mTor抑制介导的自噬对延缓衰老起到了较为明显的作用。
2.1.1 mTor和骨骼肌衰老 骨骼肌是维持其他组织蛋白质合成氨基酸的重要仓库,它在维持其他组织的蛋白质合成方面发挥着重要作用,肌肉的质量往往与对压力和重大疾病的不良反应有关[22]。Tor是一种促进骨骼肌细胞中蛋白质合成的激酶。对肌肉中mTor信号的研究表明,肌肉肥大与mTorC1的激活有关[23]。氨基酸和运动是激活Tor的主要合成代谢刺激,通过激活Tor来促进蛋白质的合成[24]。与此同时,亮氨酸作为信号分子也可以通过激活mTorC1直接在肌肉水平上发挥作用,从而起到保护和恢复肌肉质量的作用[24]。通过对人体骨骼肌的研究发现,mTor在氨基酸喂养或肌肉收缩等刺激下激活,随后会转位到细胞膜上[24]。mTorC1是人体骨骼肌在同化刺激后转位的主要mTor复合物,而mTorC2与细胞膜相关[25]。mTorC1被招募到溶酶体膜上对激活其在氨基酸作用下的激酶活性非常重要[26-27]。
保存或恢复肌肉质量对健康的老龄化至关重要,研究表明衰老与肌肉抵抗合成代谢作用有关,老年人骨骼肌质量和功能的逐渐丧失的情况,通常被称为“肌少症”[28]。然而,对大鼠的研究表明,mTorC1的激活在衰老的肌肉中被抑制,通过mTor的信号传导减少[29]。肌肉蛋白质的合成对氨基酸的敏感性和反应性较差,该信号通路的几个组成部分磷酸化状态在基础状态下似乎较高,这种情况可能是由于特定的与年龄有关的肌肉信号受到损害或营养物质和生长因子对肌肉的输送减少所致[30]。不同的肌肉肥大模型的特点是mTorC1信号的增加[31]。这表明mTorC1在人体肌肉的蛋白质合成中起着关键的作用,当使用mTorC1抑制剂时,肌肉生长明显减少甚至完全消失[32]。研究表明,雷帕霉素通过抑制两个最特殊的mTorC1靶点——S6K1和真核转录因子4E结合蛋白
(eIF4E-binding protein 1,4E-BP1)来抑制骨骼肌[33]。除了调节蛋白质合成的增加外,一些报告还将mTorC1信号与蛋白质分解联系起来[16]。早期的报告表明,在分解代谢条件下,成人骨骼肌自噬通量的增强主要是由叉头转录因子O(forkhead box O,FOXO)调节的[33-34];相反,基础自噬是由mTorC1调节的[23]。上述的 mTor所述的信号通路涉及各种疾病的发展,包括癌症、神经退行性疾病和心血管疾病[35]。根据上述结果不难看出,mTor的激活与氨基酸、运动及亮氨酸有关,在延缓肌肉老化、肌肉生长过程中具有导向作用。
2.1.2 mTor和心脏衰老 近年来的研究发现,心血管疾病在全世界造成了越来越严峻的负面影响,而运动已被证明可以预防心血管疾病[36]。研究表明,定期的有氧运动和肌肉力量训练可以有效地预防心血管疾病的发生和发展,并能缓解和改善心力衰竭患者的心功能[37]。mTor是磷酸肌醇3激酶(Phosphoinositol 3 kinase,PI3K)/Akt信号通路下游的丝氨酸/苏氨酸激酶成分[38],是心肌细胞中mRNA翻译和细胞生长的关键调节因子[39]。mTorC1在心肌细胞肥大中起重要作用,雷帕霉素抑制哺乳动物mTorC1可改善衰老和心力衰竭心脏的功能[40]。研究表明,PI3K/Akt/Tor信号通路的激活可能导致心脏肥大的发生[41]。去甲肾上腺素可以激活S6K1并通过mTorC1和可能的PI3K促进心肌细胞肥大[42]。mTorC2促进心肌细胞的发育和生存[43]。此前的报告称mTorC2通过Rictor/mTorC2的破坏对巨噬细胞刺激1的活性进行负向调节,巨噬细胞刺激1的激活可以促进心脏扩张、改善心脏功能障碍以及使心脏适应压力过载的影响[44]。mTorC2的下游底物是由血清和糖皮质激素反应性激酶1组成[45],它们在心力衰竭的早期阶段具有高活性,并在β1肾上腺素能接受器介导的自噬抑制中起作用[46]。mTorC2心肌细胞特异性Rictor敲除的小鼠在6个月大时表现出心脏异常,包括心脏扩张、心脏纤维化和因压力过载而加重的心力衰竭[47]。此外,心脏α1肾上腺素能通过mTor实现介导受体和葡萄糖摄取和心脏保护[42]。
衰老过程中的心脏肥大和心力衰竭都可以通过热量限制和雷帕霉素来改善[48]。雷帕霉素可以防止横断主动脉收缩和心肌病遗传模型中的病理性肥厚和心力衰竭[49]。雷帕霉素在小鼠肥大和心力衰竭模型中的有益作用伴随着对激活的磷酸化核糖体S6蛋白(phosphorylation of ribosomal protein S6,rpS6)和真核细胞起始因子4E(eukaryotic initiation factor 4e,eIF4E)的抑制,它们在压力过载时被磷酸化[50]。然而,通过对抑制mTor小鼠的研究发现,mTor被抑制时会对小鼠产生不利的影响,泛在或心脏特异性mTor有目的化缺失对小鼠来说是致命的,这表明mTor在胚胎生长和增殖以及心脏发育中起到重要作用[51-52]。心脏特异性消融Raptor会抑制心脏的适应性肥大并导致心脏衰竭[53]。此外,4E-BP1的积累也会引起成年小鼠心肌中的mTor消融,从而导致致命的扩张型心肌病[54]。
mTorC1的另一个重要的下游通路4EBP已被证明可以介导热量限制和mTor抑制,对果蝇心脏衰老的有益影响[55]。雷帕霉素已被证明可以抑制血管紧张素Ⅱ诱导的心肌细胞的蛋白质合成[56],恢复和逆转心脏衰老[48],抑制和逆转横断主动脉收缩诱导的心脏肥大[57],并改善收缩功能,废除肥大和心力衰竭小鼠的左心室纤维化。因此,mTor的抑制对于心脏抗衰老、心脏发育及心脏功能的改善具有积极的作用,但心脏mTor缺失可能会导致多种心脏发育相关疾病,甚至导致死亡。
2.2 mTor和运动 mTor参与的运动过程,见图4。
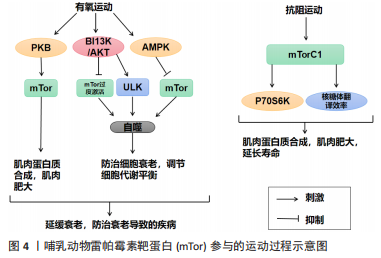
运动通过生理和生化机制包括内啡肽、线粒体、雷帕霉素的哺乳动物目标、神经递质和下丘脑-垂体-肾上腺轴及通过产热假说,对焦虑、压力和抑郁等情绪状态产生积极影响[58]。运动对Tor有激活作用。研究表明,骨骼肌纤维中的mTor信号在维持适当的纤维神经支配、维护肌纤维和mTor神经元的神经肌肉接头结构方面起着关键作用[11]。此外,运动对大多数影响神经肌肉接头的病变都有积极作用,运动的这些有益作用有一部分是通过运动中和运动后充分激活骨骼肌中的mTorC1而实现的[11]。同时,脑源性神经营养因子的表达也可以通过运动得到改善[12],脑源性神经营养因子可能是促进mTor通路激活的一个机制目标[59]。在mTor通路形成过程中,轴突再生和脊柱的突触传递起着关键作用[60-61]。
越来越多的证据表明,外周组织的运动可以有效激活mTor通路,但其核心作用仍不清楚[62]。然而,并不是所有的运动都能激活mTor通路,mTor的功能是“能量传感器”,只有在细胞代谢率高的情况下才能被激活[63]。对小鼠的研究表明,mTor通路在小鼠mTor皮质中被激活,以增强突触的发生。运动增强了第5层锥体神经元的突触后兴奋性和神经元活动,并增强了小鼠mTor皮质的轴突髓鞘形成,而这些结构和功能的变化都依赖于 mTor的激活[64]。运动时激活雷帕霉素敏感信号不仅是必要的,而且足以诱发肌肉肥大[65]。mTorC1通过磷酸化两个下游信号蛋白,即真核启动因子4E-BP1和p70核糖体S6激酶(protein 70 S6 kinase,p70S6K)来增加蛋白质的合成率,从而提高核糖体翻译的效率[66]。因此,mTorC1通路在促进蛋白质合成和随后的肌肉肥大方面起着重要作用。运动增加了能量需求,随后通过mTor的激活来满足。研究表明,mTor在依赖于mTor的皮质重塑中起着关键作用,运动激活了mTor并增强了小鼠mTor皮质的突触后密度,运动诱导的mTor的激活增强了突触的传递[64]。在体内,运动增强的神经元活动需要mTor的激活,运动通过激活mTor的形成增加轴突髓鞘的形成[64]。
由此可知,在运动过程中,只有到达一定强度才可以激活mTor,与此同时被激活的mTor对于运动过程也有促进作用,多体现于突触传递和神经活动的增强,进而对改善运动技能的学习也产生了积极的影响。此外,mTorC1是一个强有力的肌肉肥大调节器[67],因为它在影响翻译活动和能力方面具有双重作用[68]。最近的研究表明,运动对心血管系统的有益影响不仅适用于年轻和健康的人[69]。它也适用于有明显心血管危险因素或明显心血管疾病的患者,并且在停止训练后似乎可以恢复[70]。最重要的是,运动似乎可以防止心肌缺血再灌注损伤[71]。
2.2.1 mTor和有氧运动 有氧运动对延缓骨骼肌衰老具有深远的影响。通过对大鼠的研究表明,有氧运动不仅增加骨骼肌氧化能力,而且增加骨骼肌蛋白质合成率和防止与年龄相关的肌肉萎缩[72]。综合这些发现表明,有氧运动可以通过增加蛋白质合成率来减少随着年龄增长而流失的肌肉量。因此,有氧运动可能为老年人提供比抗阻运动更可行的刺激骨骼肌蛋白质合成和肌肉肥大的干预措施[73]。有氧运动通过减少内皮功能障碍、改善肌肉灌注和肌肉组织的氨基酸可用性以及增强 Akt/mTor 信号通路和肌肉蛋白的激活来克服与年龄相关的肌肉蛋白质合成胰岛素抵抗合成[74]。对老年小鼠进行3个月的自愿车轮跑步发现,有氧运动增加了Ser473 上的 PKB 磷酸化以及老年小鼠腓肠肌肌肉中 PKB 和 mTor 的总水平[75]。PKB被认为通过磷酸化mTor 的羧基末端调节结构域的磷酸化雷帕霉素靶蛋白抗体(phospho-mTor,Ser 2448)来激活mTor,因此 PKB 的表达和/或磷酸化增加可能导致激活mTor的能力更强[76]。此外,有氧运动提高了老年小鼠身上比目鱼肌的蛋白质合成率[77]。这些发现是有氧运动增强 PKB/mTor 信号通路的第一个证据,并且可能对骨骼蛋白合成随年龄增长的调节具有重要意义。
有氧运动对心肌腺苷酸活化蛋白激酶(AMP-activated protein kinase,AMPK)具有激活作用,被激活的AMPK可以有效抑制mTor活性,从而改善心肌细胞的自噬能力,防治心肌细胞的衰老性功能衰退[77]。除此之外,对阿尔茨海默症小鼠的研究表明有氧运动还可上调大脑皮质 PI3K/Akt 的活性,抑制mTor的过度激活,进而提高自噬水平[78]。同时研究发现,当有氧运动提高自噬障碍小鼠海马内 PI3K/Akt的活性时,可通过下调mTor的过度激活,上调ULK1的表达,启动自噬[79]。通过建立饥饿诱导自噬增加的小鼠模型,发现有氧运动在激活其骨骼肌中mTor的活性时,抑制自噬的过度激活[80]。总之,在有氧运动中mTor的激活始终对自噬的活性起到抑制作用,而通过抑制mTor来激活自噬可能是延缓衰老的有效途径之一。
2.2.2 mTor和抗阻运动 抗阻运动是增加肌肉力量和肌肉肥大的有效策略,后者最终由运动引起的肌肉蛋白质合成和净蛋白质平衡增加所导致[81]。研究表明,抗阻运动可以增加老年人的肌肉力量以及骨骼肌蛋白质合成率[82]。抗阻运动似乎可以通过增加骨骼肌中蛋白质的合成速度来预防与年龄相关的肌肉萎缩[83]。促进运动适应性反应的两种关键信号蛋白包括AMPK和mTor的机制靶标[83]。AMPK 通过复合物的直接磷酸化以及TSC1/2复合物的磷酸化和激活,从而在机制上阻断mTORC1信号传导,上调骨骼肌自噬维持细胞代谢平衡,这是一种mTORC1信号的上游抑制剂[84]。抗阻运动对mTor通路及AMPK通路都有激活作用,即使mTorC1和AMPK都可以响应抗阻运动而被激活,但它们的激活可能是不同步的,一旦AMPK信号消退,mTorC1就会被激活。在此过程中,mTor的激活可能是由于足够强度的抗阻运动导致的胰岛素浓度上升[84]。mTor通路的激活会更有利于提升抗阻训练质量、促进骨骼肌肥大[83]。抗阻训练后的肌肉肥大与mTor的下游靶点S6K磷酸化增加有关,协同肌消融诱导的肌肉肥大受S6K活性影响[85]。mTor底物的磷酸化(作为mTor活性的代表)与抗阻运动在禁食状态下增加肌肉蛋白质合成的能力相一致,表明mTor在抗阻运动后被激活[86]。对大鼠的研究表明,大鼠进行一次类似阻力运动的收缩可以诱导mTor下游信号蛋白 p70S6K 磷酸化增加,并且p70S6K磷酸化增加的幅度与肥大的诱导高度相关[87]。
反复进行的抗阻运动通过激活肌肉蛋白合成诱导骨骼肌肥大[88]。翻译调控被认为在抗阻运动诱导的肌肉蛋白质合成激活中发挥核心作用[89]。翻译活动由翻译效率和翻译能力决定[89]。据报道,每个核糖体的翻译效率主要受mTorC1信号活性的机制靶标调节,翻译能力主要取决于每个细胞单位的核糖体数量[90]。最近的研究表明,抗阻运动激活核糖体生物发生并导致骨骼肌中核糖体含量增加[89]。抗阻运动诱导的核糖体含量增加被认为通过增加静息条件下的肌肉蛋白质合成促进肌肉肥大[90]。蛋白质翻译的起始步骤是影响翻译效率的限速步骤,mTorC1活性对于起始翻译很重要[90]。研究表明p70S6K磷酸化水平是影响mTorC1活性的有效指标,与肌肉肥大的程度相关[90]。此外,抑制mTorC1活性会减少抗阻运动诱导的肌肉蛋白质合成激活和肌肉肥大的程度[89]。因此,上述结果表明mTorC1活性通过激活大量的核糖体来提升翻译效率,在抗阻运动诱导的肌肉蛋白合成加速中起着核心作用。此外,热量限制可有效减少阻力运动期间的脂肪量,但碳水化合物充足的高热量饮食可能是诱导肌肉肥大的最佳选择[91]。
2.3 mTor、饮食习惯和衰老 mTor参与高脂肪/高盐饮食的过程见图5。
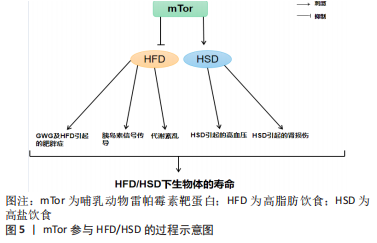
长寿和新陈代谢健康受到饮食中摄入营养物质的影响[92]。哺乳动物细胞的生长和细胞分裂依赖于氨基酸、葡萄糖和脂质,而mTor是这3种饮食宏量营养素的共同传感器[93]。mTor参与调节肝脏中的生酮作用、生脂作用和糖原代谢[94]。β-肾上腺素能受体信号被确定为小鼠肝脏中mTorC1的一个新的正向调节器[95]。对小鼠的研究表明,在高钙饮食期间,β-肾上腺素能受体信号的慢性激活可能通过mTorC1的过度激活促进肝脏中的脂质沉积[95]。
过量的营养对身体的影响并不比正常饮食更积极。营养过剩和选择性胰岛素抵抗对mTorC1的过度激活导致非酒精性脂肪肝[96]。营养过剩的环境长期激活mTorC1,导致S6K1的持续刺激和S6K1介导的磷酸化和胰岛素受体底物1的降解[97]。这种持续的刺激损害了PI3K/AKT信号通路,使胰岛素信号脱敏,从而使细胞对胰岛素产生抗性[98]。长期的高营养水平可以使mTorC1保持活跃,维持胰岛素受体基因(insulin receptor,INSR)的负反馈环,导致全身性的胰岛素不敏感[99]。对小鼠的研究表明,由脂肪分解和代谢率升高引起的mTorC1下游靶点S6K1的系统失活可以防止饮食引起的雄性小鼠的胰岛素抵抗和肥胖,并延长雌性小鼠的寿命[100]。与此相一致的是,4E-BP1表达量增加对mRNA翻译的抑制作用被mTorC1抵消,以防止衰老引起的和高脂肪饮食引起的肥胖和代谢紊乱,仅出现在雄性小鼠中[101]。然而,4E-BP1和4E-BP2的联合中断产生了相反的效果,这些小鼠出现了肥胖的情况,并对高脂肪饮食引起的肥胖和胰岛素抵抗的敏感性增加[102]。总之,mTor的激活对不良饮食导致的肥胖、衰老等负面影响具有良好的调节作用。此外,抑制mTorC1信号传导的积极作用可能具有性别特征。
2.3.1 mTor、高脂饮食和衰老 高脂肪饮食威胁到健康和生命,其引起的肥胖是果蝇及人类心脏和体液过早衰老和死亡的原因[103]。对怀孕小鼠的研究表明,与喂食正常饮食的对照组相比,怀孕期间喂食高脂肪饮食的小鼠不仅自己体质量增加,而且其胎儿的体质量也增加了[104]。妊娠期肥胖和妊娠期体质量过度增加(gestational weight gain,GWG)对母亲和婴儿来说都是危险的,甚至是致命的[104]。GWG是一项重要的健康指标,与正常体质量的妇女相比,肥胖妇女发生流产、妊娠期糖尿病、先兆子痫和死胎的风险增加[105]。二甲双胍可以减少高脂血症母猪的胎儿体质量增加,而抑制胎盘mTor是有效减少胎儿体质量增加的机制之一[106]。高脂肪饮食处理的小鼠在许多组织(包括胰腺)中具有较高的mTorC1信号,这可能是由于循环中的胰岛素、氨基酸和促炎症细胞因子水平的增加而导致的[107]。
mTor作用于调节能量平衡的不同蛋白复合物(mTorC1和mTorC2),含有mTor相互作用蛋白(DEPTOR)的DEP域是这些复合物的一部分,已知其可以抑制mTorC1的功能,从而减少mTorC1的负反馈,促进胰岛素信号传导[107]。mTor相互作用蛋白在能量平衡和系统代谢的调节中起到重要作用,其过表达在预防高脂肪饮食引起的肥胖、改善糖代谢和预防代谢紊乱的发展方面起着非常重要的作用[107]。研究表明,mTor可以延长肥胖症的发展时间。此外,mTor被证明可以延长高脂肪饮食下的肥胖小鼠的寿命,而间歇性给予雷帕霉素可以显著提高肥胖小鼠的生存率[108]。对高脂肪饮食果蝇的研究表明,雷帕霉素通过Tor通路的TorC1分支改变自噬和翻译来延长寿命,雷帕霉素通过弱胰岛素/Igf信号通路(IGF-1 signaling ,IIS)延长突变体的寿命,并通过饮食限制使果蝇的寿命最大化[109]。这项研究的结果表明,mTor可以用来增加高脂肪饮食果蝇的寿命。因此,很明显,当mTor被抑制时,可以更好地控制高脂肪饮食的负面影响,而当mTor作用的蛋白mTor相互作用蛋白被过度表达时,可以防止高脂肪饮食引起的肥胖,改善高脂肪饮食引起的代谢问题。
2.3.2 mTor、高盐饮食和衰老 每天摄入太多的盐会对身体造成很大的伤害。研究表明,高钠或盐的摄入可导致多种慢性疾病,包括高血压、心力衰竭、慢性肾脏疾病、脑卒中、心血管疾病,有研究显示高盐饮食通过上调心脏病的风险来增加死亡率[110]。对衰老果蝇的研究表明,高盐饮食通过上调盐的表达和抑制dFOXO/SOD通路,对与年龄相关的攀爬能力和死亡率产生了负面影响,并且dFOXO/SOD通路活性的增加在介导耐力运动对低盐耐力的抵抗中起着关键作用,导致衰老果蝇的攀爬能力和寿命受损[111]。高盐(4.0%NaCl)饮食摄入诱导肌肉细胞的自噬作用[112]。在对小鼠的研究中发现,在高盐饮食下小鼠产生尿素作为替代渗透剂,促进Na+排泄,同时避免水分流失,这种新陈代谢的渗透保护反应涉及到肌肉中自噬活动的增加[113]。
饮食中摄入的高盐会激活mTorC1,而用雷帕霉素抑制这一通路可以缓解Dahl盐敏感(dahl salt-sensitive,SS)大鼠的盐诱导高血压及肾损伤[113]。NOX4(肾脏中最丰富的NOX异构体)衍生的过氧化氢是mTorC1激活的一个重要决定因素,在高盐饮食的SS大鼠的通路失调中起着关键作用,是SS大鼠盐分过高和进行性肾脏损伤得到改善的关键[113-114]。NOX4产生的过氧化氢作为mTorC1活性的上游调节器,在维持4.0%NaCl的大鼠中,mTorC1被生理相关浓度的过氧化氢强烈激活,但在该细胞类型中被超生理浓度的过氧化氢抑制,独立于过氧化氢的浓度,这也是为什么雷帕霉素复合激酶抑制剂的哺乳动物靶点联合抑制mTorC1和mTorC2,完全防止和逆转了盐诱导的SS大鼠高血压[113]。SS大鼠对盐诱导高血压的反应比单独使用雷帕霉素抑制mTorC1所观察到的要大得多[66],这表明mTorC2对显著保护SS大鼠免受盐诱导高血压的影响有重要贡献[114]。对果蝇的研究表明,飞行运动可以有效改善野生果蝇对高脂肪饮食和高盐饮食的偏好和不良影响,经常性的有氧运动可以抑制饮食对果蝇线粒体功能、生理和摄食行为的不良影响[115]。因此,高盐饮食会诱发肌肉自噬活动的发生并激活mTorC1,mTorC1和mTorC2可有效治疗高盐饮食引起的高血压,运动可以很好地逆转和控制高盐饮食的不利影响,但运动和高盐饮食对mTor产生的作用尚不清楚。

| [1] ESCOBAR KA, COLE NH, MERIER CM, et al. Autophagy and aging: Maintaining the proteome through exercise and caloric restriction. Aging Cell. 2019;18(1):e12876. [2] SAXTON RA, SABATINI DM. mTOR Signaling in Growth, Metabolism, and Disease. Cell. 2017;168(6):960-976. [3] OGIENKO AA, OMELINA ES, BYLINO OV, et al. Drosophila as a Model Organism to Study Basic Mechanisms of Longevity. Int J Mol Sci. 2022;23(19):11244. [4] CHENG L, QIU L, ZHANG R, et al. Functional variant of MTOR rs2536 and survival of Chinese gastric cancer patients. Int J Cancer. 2019;144(2):251-262. [5] APELO SIA, LAMMING DW. Rapamycin: An InhibiTOR of Aging Emerges From the Soil of Easter Island. J Gerontol A Biol Sci Med Sci. 2016;71(7):841-849. [6] AZAR A, LAWRENCE I, JOFRE S, et al. Distinct patterns of gene expression in human cardiac fibroblasts exposed to rapamycin treatment or methionine restriction. Ann N Y Acad Sci. 2018;1418(1):95-105. [7] LIU H, DING J, KOHNLEIN K, et al. The GID ubiquitin ligase complex is a regulator of AMPK activity and organismal lifespan. Autophagy. 2020;16(9):1618-1634. [8] WEICHHART T. mTOR as Regulator of Lifespan,Aging,and Cellular Senescence:A Mini-Review. Gerontology. 2018;64(2):127-134. [9] MANNICK J, GIUDICE GD, LATTANZI M, et al. mTOR inhibition improves immune function in the elderly. Gerontologist. 2016;6(268):686-686. [10] FIGUEROA CM, LUNN JE. A Tale of Two Sugars: Trehalose 6-Phosphate and Sucrose(1 OPEN). Plant Physiology. 2016;172(1):7-27. [11] BARALDO M, GEREMIA A, PIRAZZINI M, et al. Skeletal muscle mTORC1 regulates neuromuscular junction stability. J Cachexia Sarcopenia Muscle. 2020;11(1):208-225. [12] HEISZ JJ, CLARK IB, BONIN K, et al. The Effects of Physical Exercise and Cognitive Training on Memory and Neurotrophic Factors. J Cogn Neurosci. 2017;29(11): 1895-1907. [13] GUO P, MA X, ZHAO W, et al. TRIM31 is upregulated in hepatocellular carcinoma and promotes disease progression by inducing ubiquitination of TSC1-TSC2 complex. Oncogene. 2018;37(4):478-488. [14] BIN JM, NADEEM MS, GILANI SJ, et al. Genes and Longevity of Lifespan. Int J Mol Sci. 2022;23(3):1499. [15] CHENG MC, KATHARE PK, PAIK I, et al. Phytochrome Signaling Networks. Annu Rev Plant Biol. 2021;72:217-244. [16] WILSON RH, MORGAN TJ, MACKAY TFC. High-resolution mapping of quantitative trait loci affecting increased life span in Drosophila melanogaste. Genetics. 2006; 173(3):1455-1463. [17] JOHNSTONE C, CHAVES-POZO E. Antigen Presentation and Autophagy in Teleost Adaptive Immunity. Int J Mol Sci. 2022;23(9):4899. [18] ZHANG FY, ZHAO SH, YAN WJ, et al . Branched Chain Amino Acids Cause Liver Injury in Obese/Diabetic Mice by Promoting Adipocyte Lipolysis and Inhibiting Hepatic Autophagy. Ebiomedicine. 2016;13:157-167. [19] KITADA M, OGURA Y, SUZUKI T, et al. A very-low-protein diet ameliorates advanced diabetic nephropathy through autophagy induction by suppression of the mTORC1 pathway in Wistar fatty rats, an animal model of type 2 diabetes and obesity. Diabetologia. 2016;59(6):1307-1317. [20] SPAULDING HR, YAN Z. AMPK and the Adaptation to Exercise. Annu Rev Physiol. 2022;10(84):209-227. [21] ADEVA MM, FERNANDEZ C, LOPEZ PY, et al. The effects of glucagon and the target of rapamycin (TOR) on skeletal muscle protein synthesis and age-dependent sarcopenia in humans. Clin Nutr ESPEN. 2021;44:15-25. [22] HAM DJ, BORSCH A, CHOJNOWSKA K, et al. Distinct and additive effects of calorie restriction and rapamycin in aging skeletal muscle. Nat Commun. 2022; 13(1):2025. [23] MENG D, YANG Q, WANG H, et al. Glutamine and asparagine activate mTORC1 independently of Rag GTPases. J Biol Chem. 2020;295(10):2890-2899. [24] BLAGOSKLONNY MV. Rapamycin for longevity: opinion article. Aging (Albany NY). 2019;11(19):8048-8067. [25] EVAVOLD CL,HAFNER BI,DEVANT P,et al. Control of gasdermin D oligomerization and pyroptosis by the Ragulator-Rag-mTORC1 pathway. Cell. 2021;184(17):4495-4511. [26] JOANISSE S, NEDERVEEN JP, SNIJDER T, et al. Skeletal Muscle Regeneration, Repair and Remodelling in Aging:The Importance of Muscle Stem Cells and Vascularization. Gerontology. 2017;63(1):91-100. [27] ANDREOU AP, LEESE C, GRECO R, et al. Double-Binding Botulinum Molecule with Reduced Muscle Paralysis: Evaluation in In Vitro and In Vivo Models of Migraine. Neurotherapeutics. 2021;18(1):556-568. [28] DE BANDT JP. Leucine and Mammalian Target of Rapamycin-Dependent Activation of Muscle Protein Synthesis in Aging. J Nutr. 2016;146(12): 2616-2624. [29] PEREIRA MG, DYAR KA, NOGARA, et al. Comparative Analysis of Muscle Hypertrophy Models Reveals Divergent Gene Transcription Profiles and Points to Translational Regulation of Muscle Growth through Increased mTOR Signaling. Front Physiol. 2017;8:968. [30] BLOOMGARDEN Z. Diabetes and branched-chain amino acids: What is the link? J Diabetes. 2018;10(5):350-352. [31] MARABITA M, BARALDO M, SOLAGNA F, et al. S6K1 Is Required for Increasing Skeletal Muscle Force during Hypertrophy. Cell Rep. 2016; 17(2):501-513. [32] MILAN G, ROMANELLO V, PESCATORE F, et al. Regulation of autophagy and the ubiquitin-proteasome system by the FoxO transcriptional network during muscle atrophy. Nat Commun. 2015;6:6670. [33] LIU GY, SABATINI DM. mTOR at the nexus of nutrition, growth, ageing and disease. Nat Rev Mol Cell Biol. 2020;21(4):183-203. [34] SUNG YL, WU CE, SYU JY, et al. Effects of long-term exercise on arrhythmogenesis in aged hypertensive rats. Comput Biol Med. 2018;102:390-395. [35] SCHUTTLER D, CLAUSS S, WECKBACH LT, et al. Molecular Mechanisms of Cardiac Remodeling and Regeneration in Physical Exercise. Cells. 2019;8(10): 1128. [36] XU F, NA LX, LI YF, et al. Roles of the PI3K/AKT/mTOR signalling pathways in neurodegenerative diseases and tumours. Cell Biosci. 2020;10(1):54. [37] YIN SS, LIU L, GAN WJ. The Roles of Post-Translational Modifications on mTOR Signaling. Int J Mol Sci. 2021;22(4):1784. [38] DAI DF, LIU Y, BASISTY N, et al. Differential effects of various genetic mouse models of the mechanistic target of rapamycin complex I inhibition on heart failure. Geroscience. 2019;41(6):847-860. [39] BA L, GAO J, CHEN Y, et al. Allicin attenuates pathological cardiac hypertrophy by inhibiting autophagy via activation of PI3K/Akt/mTOR and MAPK/ERK/mTOR signaling pathways. Phytomedicine. 2019;58:152765. [40] CHIA LY, WVANS BA, MUKAIDA S, et al. Adrenoceptor regulation of the mechanistic target of rapamycin in muscle and adipose tissue. Br J Pharmacol. 2019;176(14):2433-2448. [41] GONZALEZ-TERAN B, LOPEZ JA, RODRIGUEZ, et al. p38 gamma and delta promote heart hypertrophy by targeting the mTOR-inhibitory protein DEPTOR for degradation. Nat Commun. 2016;7:10477. [42] SCIARRETTA S, ZHAI P, MAEJIMA Y, et al. mTORC2 Regulates Cardiac Response to Stress by Inhibiting MST1. Cell Rep. 2015;11(1):125-136. [43] YANO T, FERLITO M, APONTE A, et al. Pivotal Role of mTORC2 and Involvement of Ribosomal Protein S6 in Cardioprotective Signaling. Circ Res. 2014;114(8):1268-1280. [44] BAGO R, MALIK N, MUNSON MJ, et al. Characterization of IIPS34-IN1,a selective inhibitor of Vps34, reveals that the phosphatidylinositol 3-phosphate-binding SGK3 protein kinase is a downstream target of class Ill phosphoinositide 3-kinase. Biochem Jl. 2014;463(3):413-427. [45] DAI DF, KARUNADHARMA PP, CHIAO YA, et al. Altered proteome turnover and remodeling by short-term caloric restriction or rapamycin rejuvenate the aging heart. Aging Cell. 2014;13(3):529-539. [46] TONG WH, OLLIVIERRE H, NOGUCHI A, et al. Hyperactivation of mTOR and AKT in a cardiac hypertrophy animal model of Friedreich ataxia. Heliyon. 2022; 8(8):e10371. [47] WANG L, HAO H, WANG J, et al. Decreased autophagy:a major factor for cardiomyocyte death induced by beta(1)-adrenoceptor autoantibodies. Cell Death Dis. 2015;6(8):e1862. [48] GOGIRAJU R, HUBERT A, FAHRER J, et al. Endothelial Leptin Receptor Deletion Promotes Cardiac Autophagy and Angiogenesis Following Pressure Overload by Suppressing Akt/mTOR Signaling. Circ Heart Fail. 2019;12(1): e005622. [49] LARABI A, BARNICH N, NGUYEN HTT. New insights into the interplay between autophagy, gut microbiota and inflammatory responses in IBD. Autophagy. 2020;16(1):38-51. [50] GANASSI M, ZAMMIT PS. Involvement of muscle satellite cell dysfunction in neuromuscular disorders: Expanding the portfolio of satellite cell-opathies. Eur J Transl Myol. 2022;32(1):10064. [51] SCIARRETTA S, FORTE M, FRATI G, et al. New Insights Into the Role of mTOR Signaling in the Cardiovascular System. Circ Res. 2018;122(3):489-505. [52] CHAULIN AM. Review of Recent Laboratory and Experimental Data on Cardiotoxicity of Statins. J Cardiovasc Dev Dis. 2022;9(11):403. [53] PENG T, DING M, YAN H, et al. Exercise Training Upregulates Cardiac mtp Expression in Drosophila melanogaster with HFD to Improve Cardiac Dysfunction and Abnormal Lipid Metabolism. Biology (Basel). 2022;11(12):1745. [54] SADOSHIMA J, QIU ZH, MORGAN JP, et al. Angiotensin-II and other hypertrophic stimuli mediated byg-protein-coupled receptors activate tyrosine kinase, mitogen-activated protein-kinase and 90-KD S6 kinase in cardiac myocytes-The critical role of Ca2+-dependent signaling. Circ Res. 1995;76(1):1-15. [55] SELVARANI R, MOHAMMED S, RICHARDSON A. Effect of rapamycin on aging and age-related diseases-past and future. Geroscience. 2021;43(3):1135-1158. [56] KENNEDY BK, LAMMING DW. The Mechanistic Target of Rapamycin: The Grand ConducTOR of Metabolism and Aging. Cell Metabolism. 2016;23(6):990-1003. [57] KIM J, GUAN KL. mTOR as a central hub of nutrient signalling and cell growth. Nature Cell Biology. 2019;21(1):63-71. [58] MIKKELSEN K, STOJANOVSKA L, POLENAKOVIC M, et al. Exercise and mental health. Maturitas. 2017;106:48-56. [59] MOURO FM, ROMBO DM, DIAS RB, et al. Adenosine A2A receptors facilitate synaptic NMDA currents in CA1 pyramidal neurons. Br J Pharmacol. 2018; 175(23):4386-4397. [60] MOLONEY PB, CAVALLERI GL, DELANTY N. Epilepsy in the mTORopathies: opportunities for precision medicine. Brain Commun. 2021;3(4):fcab222. [61] RITT M, RITT JI, SIEBER CC, et al. Comparing the predictive accuracy of frailty,comorbidity,and disability for mortality:a 1-year follow-up in patients hospitalized in geriatric wards. Clin Interv Aging. 2017;12:293-304. [62] CHEN K, ZHENG Y, WEI J, et al. Exercise training improves motor skill learning via selective activation of mTOR. Sci Adv. 2019;5(7):eaaw1888. [63] TOH P, NICHOLSON JL, VETTER AM, et al. Selenium in Bodily Homeostasis:Hypothalamus,Hormones, and Highways of Communication. Int J Mol Sci. 2022;23(23):15445. [64] KUMAR V, WOLLNER C, KURTH T, et al. Inhibition of Mammalian Target of Rapamycin Complex 1 Attenuates Salt-Induced Hypertension and Kidney Injury in Dahl Salt-Sensitive Rats. Hypertension. 2017;70(4):813-821. [65] LASHGARI NA, ROUDSARI NM, ZADEH SST, et al. Statins block mammalian target of rapamycin pathway: a possible novel therapeutic strategy for inflammatory, malignant and neurodegenerative diseases. Inflammopharmacology. 2022;1-19. doi: 10.1007/s10787-022-01077-w. [66] WEST DWD, BAEHR LM, MARCOTTE GR, et al. Acute resistance exercise activates rapamycin-sensitive and insensitive mechanisms that control translational activity and capacity in skeletal muscle. J Physiol. 2016;594(2):453-468. [67] VACCARINO V, SULLIVAN S, HAMMADAH M, et al. Mental Stress-Induced-Myocardial Ischemia in Young Patients With Recent Myocardial Infarction: Sex Differences and Mechanisms. Circulation. 2018;137(8):794-805. [68] MENGHINI R, CASAGRANDE V, LULIANI G, et al. Metabolic aspects of cardiovascular diseases: Is FoxO1 a player or a target? Int J Biochem Cell Biol. 2020;118:105659. [69] WANG B, XU M, LI W, et al. Aerobic exercise protects against pressure overload-induced cardiac dysfunction and hypertrophy via β3-AR-nNOS-NO activation. PLoS One. 2017;12(6):e0179648. [70] KITADA M, OGURA Y, MONNO I, et al. The impact of dietary protein intake on longevity and metabolic health. Ebiomedicine. 2019;43:632-640. [71] MENON D, SALLOUM D, BERNFELD E, et al. Lipid sensing by mTOR complexes via de novo synthesis of phosphatidic acid. J Biol Chem. 2017;292(15):6303-6311. [72] REYNOLDS TH 4th, REID P, LARKIN LM, et al. Effects of aerobic exercise training on the protein kinase B (PKB)/mammalian target of rapamycin(mTOR) signaling pathway in aged skeletal muscle. Exp Gerontol. 2004;39(3):379-385. [73] XIA Q, HUANG X, HUANG J, et al. The Role of Autophagy in Skeletal Muscle Diseases. FrontPhysiol. 2021;12:638983. [74] 张雪,漆正堂,丁树哲.线粒体介导骨骼肌重塑在运动抵御癌症恶病质中的作用研究进展[J].上海体育学院学报,2021,45(1):78-85. [75] 金晶,冯祎中,黄伟,等.运动通过激活卫星细胞功能延缓和改善骨骼肌衰减症的研究进展[J].体育科学,2019,39(8):73-80. [76] LI P, MA Y, YU C, et al. Autophagy and Aging: Roles in Skeletal Muscle, Eye, Brain andHepatic Tissue. Front Cell Dev Biol. 2021;9:752962. [77] DRAKE JC, WILSON RJ, CUI D, et al. Ulk1, Not Ulk2, Is Required for Exercise Training-Induced Improvement of Insulin Response in Skeletal Muscle. Front Physiol. 2021;12:732308. [78] CHANGOTRA H, KAUR S, YADAV SS, et al. ATG5: A central autophagy regulator implicated invarious human diseases. Cell Biochem Funct. 2022;40(7):650-667. [79] MEJIAS-PENA Y, ESTEBANEZ B, RODRIGUEZ-MIGUELEZ P, et al. Impact of resistance training on the autophagy-inflammation-apoptosis crosstalk in elderly subjects. Aging (Albany NY). 2017;9(2):408-418. [80] 张鑫愉,周晓勐,牛燕媚,等.mTOR 复合物在有氧运动改善小鼠骨骼肌糖代谢过程中的作用[J].中国运动医学杂志,2018,37(12):1017-1023. [81] FIGUEIREDO VC, ROBERT LA, MARKWORTH JF, et al. Impact of resistance exercise on ribosome biogenesis is acutely regulated by post-exercise recovery strategies. Physiol Rep. 2016;4(2):e12670. [82] KOTANI T, TAKEGAKI J, TAKAGI R, et al. Consecutive bouts of electrical stimulation-induced contractions alter ribosome biogenesis in rat skeletal muscles. J Appl Physiol (1985). 2019;126(6):1673-1680. [83] MOBLEY CB, HOLLAND AM, KEPHART WC, et al. Progressive resistance-loaded voluntary wheel running increases hypertrophy and differentially affects muscle protein synthesis, ribosome biogenesis,and proteolytic markers in rat muscle. J Anim Physiol Anim Nutr (Berl). 2018;102(1):317-329. [84] IMAMI K, MILEK M, BOGDANOW B, et al. Phosphorylation of the ribosomal protein RPL12/uL11 affects translation during mitosis. Molecular Cell. 2018;72(1): 84-98. [85] GOODMAN CA. Role of mTORC1 in mechanically induced increases in translation and skeletal muscle mass. J Appl Physioln. 2019;127(2):581-590. [86] GROENNEBAEK T, JESPERSEN NR, JAKOBSGAARD JE, et al. Skeletal muscle mitochondrial protein synthesis and respiration increase with low-load blood flow restricted as well as high-load resistance training. Front Physiol. 2018;9:1796. [87] HAMMARSTROM D, OFSTENG S, KOLL L, et al. Benefits of higher resistance-training volume are related to ribosome biogenesis. J Physiol. 2020;598(3): 543-565. [88] HANSSON B, OLSEN LA, NICOLL JX, et al. Skeletal muscle signaling responses to resistance exercise of the elbow extensors are not compromised by a preceding bout of aerobic exercise. Am J Physiol Regul Integr Comp Physiol. 2019;317(1):R83-R92. [89] HAUN CT, VANN CG, MOBLEY CB, et al. Pre-training skeletal muscle fiber size and predominant Fiber type best predict hypertrophic responses to 6 weeks of resistance training in previously trained young men. Front Physiol. 2019;10:297. [90] JAKOBSGAARD JE, ANDRESEN J, DE PAOLI FV, et al. Skeletal muscle phenotype signaling with ex vivo endurance-type dynamic contractions in rat muscle. J Appl Physiol. 2021;131(1):45-55. [91] JHANWAR-UNIYAL M, WAINWRIGHT JV, MOHAN AL, et al. Diverse signaling mechanisms of mTOR complexes: mTORC1 and mTORC2 in forming a formidable relationship. Adv Biol Regul. 2019;72:51-62. [92] PAPA A, PANDOLFI PP. The PTEN⁻PI3K Axis in Cancer. Biomolecules. 2019;9(4):153. [93] DEN HARTIGH LJ, GOODSPEED L, WANG SA, et al. Chronic oral rapamycin decreases adiposity, hepatic triglycerides and insulin resistance in male mice fed a diet high in sucrose and saturated fat. Exp Physiol. 2018;103(11):1469-1480. [94] BAR-TANA J. Type 2 diabetes-unmet need, unresolved pathogenesis, mTORC1-centric paradigm. Rev Endocr Metab Disord. 2020;21(4):613-629. [95] SUN J, LU H, LIANG W, et al. Endothelial TFEB (Transcription Factor EB) Improves Glucose Tolerance via Upregulation of IRS (Insulin Receptor Substrate) 1 and IRS2. Arterioscler Thromb Vasc Biol. 2021;41(2):783-795. [96] GAO W, DU X, LEI L, et al. NEFA-induced ROS impaired insulin signalling through the JNK and p38MAPK pathways in non-alcoholic steatohepatitis. J Cell Mol Med. 2018;22(7):3408-3422. [97] MOHR AE, JASBI P, BOWES DA, et al. Exploratory analysis of one versus two-day intermittent fasting protocols on the gut microbiome and plasma metabolome in adults with overweight/obesity. Front Nutr. 2022;9:1036080. [98] NATHAN N, KEPPLER-NOREUIL KM, BIESECKER LG, et al. Mosaic Disorders of the PI3K/PTEN/AKT/TSC/mTORC1 Signaling Pathway. Dermatol Clin. 2017;35(1):51-60. [99] TSAI SY, RODRIGUEZ AA, DASTIDAR SG, et al. Increased 4E-BP1 Expression Protects against Diet-Induced Obesity and Insulin Resistance in Male Mice. Cell Rep. 2016;16(7):1903-1914. [100] LE BACQUER O, COMBE K, MONTAURIER C, et al. Muscle metabolic alterations induced by genetic ablation of 4E-BP1 and 4E-BP2 in response to diet-induced obesity. Mol Nutr Food Res. 2017;61(9). doi: 10.1002/mnfr.201700128 [101] WEN D T, ZHENG L, LU K, et al. Physical exercise prevents age-related heart dysfunction induced by high-salt intake and heart salt-specific overexpression in Drosophila. Aging-Us. 2021;13(15):19542-19560. [102] VORA NL, GRACE MR, SMEESTER L, et al. Targeted Multiplex Gene Expression Profiling to Measure High-Fat Diet and Metformin Effects on Fetal Gene Expression in a Mouse Model. Reprod Sci. 2019;26(5):683-689. [103] DANIEL S, ROTEM R, KOREN G, et al. Vaginal antimycotics and the risk for spontaneous abortions. Am J Obstet Gynecol. 2018;218(6):601.e1-601.e7. [104] GRACE MR, DOTTERS-KATZ SK, ZHOU C, et al. Effect of a High-Fat Diet and Metformin on Placental mTOR Signaling in Mice. AJP Rep. 2019;9(2):e138-e143. [105] NEPSTAD I, HATFIELD KJ, GRONNINGSAETER IS, et al. The PI3K-Akt-mTOR Signaling Pathway in Human Acute Myeloid Leukemia (AML) Cells. Int J Mol Sci. 2020;21(8):2907. [106] LEONTIEVA OV, PASZKIEWICZ GM, BLAGOSKLONNY MV. Weekly administration of rapamycin improves survival and biomarkers in obese male mice on high-fat diet. Aging Cell. 2014;13(4):616-622. [107] BJEDOV I, TOIVONEN JM, KERR F, et al. Mechanisms of Life Span Extension by Rapamycin in the Fruit Fly Drosophila melanogaster. Cell Metabolism. 2010; 11(1):35-46. [108] PATEL Y, JOSEPH J. Sodium Intake and Heart Failure. Int J Mol Sci. 2020;21(24): 9474. [109] LEE JY, KENNEDY BK, LIAO CY. Mechanistic target of rapamycin signaling in mouse models of accelerated aging. J Gerontol A Biol Sci Med Sci. 2020;75(1):64-72. [110] KITADA K, DAUB S, ZHANG Y, et al. High salt intake reprioritizes osmolyte and energy metabolism for body fluid conservation. J Clin Invest. 2017;127(5):1944-1959. [111] KUMAR V, ENANS LC, KURTH T, et al. Therapeutic Suppression of mTOR (Mammalian Target of Rapamycin) Signaling Prevents and Reverses Salt-Induced Hypertension and Kidney Injury in Dahl Salt-Sensitive Rats. Hypertension. 2019; 73(3):630-639. [112] KUMAR V, KURTH T, ZHELEZNOVA NN, et al. NOX4/H2O2/mTORC1 Pathway in Salt-Induced Hypertension and Kidney Injury. Hypertension. 2020;76(1):133-143. [113] MURASHOV AK,PAK ES,LIN CT,et al. Preference and detrimental effects of high fat,sugar,and salt diet in wild-caught Drosophila simulans are reversed by flight exercise. FASEB bioAdvances. 2020;3(1):49-64. [114] GUAN B, TONG J, HAO H, et al. Bile acid coordinates microbiota homeostasis and systemic immunometabolism in cardiometabolic diseases. Acta Pharm Sin B. 2022;12(5):2129-2149. [115] KWONG E, LI Y, HYLEMON PB, et al. Bile acids and sphingosine-1-phosphate receptor 2 in hepatic lipid metabolism. Acta Pharm Sin B. 2015;5(2):151-157. |
| [1] | 刘志杨, 傅泽铤, 夏 雨, 丁海丽. 运动性骨骼肌损伤中时钟基因BMAL1与MyoD的作用[J]. 中国组织工程研究, 2024, 28(4): 510-515. |
| [2] | 朱小烽, 陈为玮, 黄 健. 母鼠高脂饮食与运动干预对雄性子代胰岛素敏感性及下丘脑弓状核的影响[J]. 中国组织工程研究, 2024, 28(4): 556-561. |
| [3] | 张 明, 王 斌, 贾 凡, 陈 杰, 唐 玮. 基于脑电图的脑机接口技术在脑卒中患者上肢运动功能康复中的应用[J]. 中国组织工程研究, 2024, 28(4): 581-586. |
| [4] | 王 娟, 王 玲, 左会武, 郑 成, 王广兰, 陈 鹏. 肌内效贴对前交叉韧带重建后康复疗效的Meta分析[J]. 中国组织工程研究, 2024, 28(4): 651-656. |
| [5] | 闫海龙, 霍江涛, 周五成, 白雪桦, 梁媛媛. 筋膜加压带在运动与康复领域的应用与机制[J]. 中国组织工程研究, 2024, 28(3): 464-471. |
| [6] | 孙 园, 王庆博, 皮亦华, 陆春敏, 徐传仪, 张 艳. 早期和晚期有氧运动对野百合碱诱导肺动脉高压大鼠右心衰竭的影响[J]. 中国组织工程研究, 2024, 28(2): 177-185. |
| [7] | 尹林伟, 黄夏荣, 屈萌艰, 杨 璐, 王金玲, 贾飞阳, 廖 阳, 周 君. 跑台运动对老年大鼠骨质疏松及wnt/β-catenin信号通路的影响[J]. 中国组织工程研究, 2024, 28(2): 231-236. |
| [8] | 戴剑松, 辛东岭, 陈钢锐. 筋膜枪与拉伸放松改善运动性肌肉疲劳的比较[J]. 中国组织工程研究, 2024, 28(2): 242-246. |
| [9] | 代新语, 闫纪红, 华凌军, 郑晓鸿. 抗阻运动改善超重肥胖人群身体成分:一项伞形综述[J]. 中国组织工程研究, 2024, 28(2): 267-271. |
| [10] | 郭项英, 彭子富, 何亦敏, 房洪波, 姜 宁. MiRNA-122在运动改善非酒精性脂肪肝中的作用[J]. 中国组织工程研究, 2024, 28(2): 272-279. |
| [11] | 王京峰, 文登台, 王士杰, 高颖晖. Atg介导的自噬、运动和骨骼肌衰老[J]. 中国组织工程研究, 2024, 28(2): 295-301. |
| [12] | 吕默然, 许文鑫, 王 迪, 李 明. 功能性训练在医疗健康领域研究的新趋势与新动态[J]. 中国组织工程研究, 2024, 28(2): 302-307. |
| [13] | 常万鹏, 张钟文, 杨钰琳, 訾 阳, 杨梦琦, 杜冰玉, 王 楠, 于少泓. 康复外骨骼机器人对脑卒中下肢运动功能障碍疗效的Meta分析[J]. 中国组织工程研究, 2024, 28(2): 321-328. |
| [14] | 杨 裴. 不同运动方式对人体 DNA 损伤、DNA 甲基化和端粒长度的影响[J]. 中国组织工程研究, 2024, 28(1): 147-152. |
| [15] | 马岁录, 何志军, 刘 涛, 李 岩, 何元旭, 何 波, 王威威, 魏晓涛. 中药单体调控“细胞自噬”防治皮瓣坏死[J]. 中国组织工程研究, 2024, 28(1): 153-158. |
1.1.1 检索人及检索时间 第一作者在 2022 年 11 月进行检索。
1.1.2 检索文献时限 2010-01-01/2022-12-31。
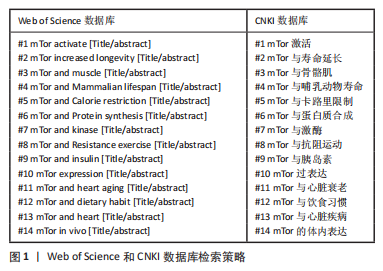
1.1.3 检索数据库 Web of Science数据库(https://www.webofscience.com),CNKI数据库(https://www.cnki.net/)。
1.1.4 检索词 mTor基因与衰老,mTor基因与心脏衰老,mTor基因与肌肉衰老,mTor基因与运动,mTor基因、运动与衰老,mTor基因与有氧运动,mTor基因与抗阻运动,mTor基因与饮食习惯,mTor基因与高脂饮食,mTor基因与高盐饮食,mTor基因、高脂饮食与衰老,mTor基因、高盐饮食与衰老;Mammalian Target of rapamycin and aging,Mammalian Target of rapamycin and heart aging,Mammalian Target of rapamycin and muscle aging,Mammalian Target of rapamycin and exercise,Mammalian Target of rapamycin and aerobic exercise,Mammalian Target of rapamycin and resistance exercise,Mammalian Target of rapamycin and dietary habits,Mammalian Target of rapamycin and high-fat diet,Mammalian Target of rapamycin and high-salt diet,Mammalian Target of rapamycin,high-fat diet and aging,Mammalian Target of rapamycin,high-salt diets and aging。
1.1.5 检索文献类型 研究原著、综述、述评、经验交流、病例报告、荟萃分析等。
1.1.6 手工检索情况 无。
1.1.7 检索策略 以使用Web of Science数据库检索文献为例,在确定文章方向之后,围绕文章关键词“老龄化,mTor,肌肉,心脏,高脂肪/盐饮食以及运动”进行关键词的组合搜索,例如输入核心词“Mammalian Target of rapamycin and aging”若搜索文献量过少,则选择减少限制词 aging 再次搜索相关领域文献;在确定文献阅读范围之后,通过文献的阅读逐步排除非相关文献。在阅读期间可利用CitespaceV 软件将所确定文献进行进一步的精简,更精准地把握相关文献与所撰写文章的契合度。具体操作步骤:在 Web of Science数据库中输入关键词“Mammalian Target of rapamycin and aging”搜索文献,经过检阅剔除会议及研究主题不相符的文献后,导出选项选择纯文本文件,记录内容选择完整记录,下载并以 download.txt的格式保存到已经建好的 date 文件夹中,利用 CitespaceV 软件将文献数据做可视化分析,锁定需要浏览的与文章相关的文献范围。
Web of Science数据库和CNKI数据库检索策略,见图1。
1.2 入选标准 纳入对应搜索关键词的所有文献;排除与核心关键词有明显出入的文献。
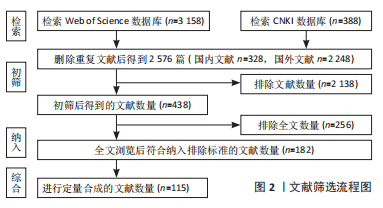
1.3 数据的提取 3 546篇删除重复文献后得到2 576篇,查看文题摘要初筛排除2 138篇,进一步全文浏览排除256篇,纳入符合标准182篇,最后排除质量不高、内容重复文献67篇,进行定量合成的文献数量为115篇(英文文献112篇,中文文献3篇)。文献筛选流程图,见图2。
 #br#
#br#
文题释义:
哺乳动物雷帕霉素靶蛋白(mammalian target of rapamycin,mTor):mTor复合物是细胞生长和代谢的中央调节剂,它整合了来自氨基酸、营养素和细胞外信号的输入,通过对这些信息进行整合加工mTor可以进一步调节下游信息最终实现生理效应,如影响细胞生长和器官发育。mTor与Tor同源,通过对诸多文献进行分析,发现包含酵母在内的多个非哺乳动物的真核生物雷帕霉素靶蛋白(target of rapamycin,Tor)也可以统称为mTor。mTor复合物(mTorC1、mTorC2):两种蛋白质复合物 mTorC1和mTorC2均包含mTor,这两种蛋白质复合物从酵母到人类在进化上是保守的。mTorC1是临床上重要的药物雷帕霉素的直接靶点,雷帕霉素变构抑制 mTorC1,而它仅间接抑制 mTorC2。mTorC1 和 mTorC2 的活性对于维持代谢稳态、预防疾病和延长健康寿命是必要的。
文章首次将mTor与运动、高脂肪/高盐饮食和衰老联系起来,旨在讨论mTor与不同因素相互串联所产生的影响,以及各因素对于mTor的影响。mTor对于衰老的积极影响是不可忽视的。mTor及其复合物mTorC1、mTorC2以或激活或抑制的形式调控心脏及肌肉衰老,在运动、高脂肪/高盐饮食中也起到关键的作用,此外mTor的激活与自噬息息相关。运动作为一种可行性的非药物疗法在延长寿命及肌肉的合成过程中占有十分重要的地位,但从长远来看,包含耐力和力量训练的同步训练如何通过mTor激活来适应肌肉肥大及有氧能力的机制也较为模糊。与此同时,运动、高脂/高盐饮食和Tor基因之间的关系也需要进一步研究。例如,进一步的研究应集中在运动是否能改善Tor过表达或敲除引起的心脏、肌肉老化,这是一个研究不足的课题。相信在不久的将来对于mTor联合运动、高脂/高盐饮食在衰老中的研究将会陆续展开,为预防和治疗衰老的机制提供一个更全面的认识。
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||