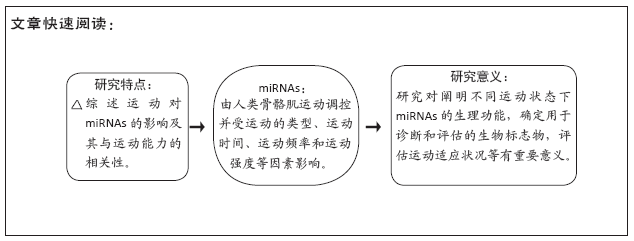
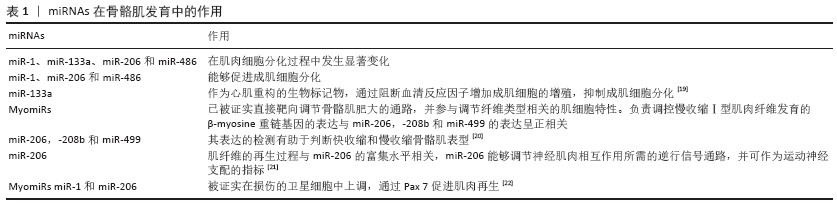
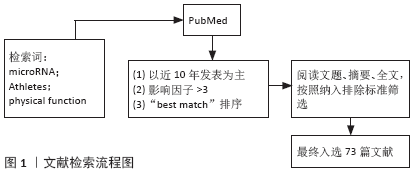
[1] NGUYEN HM, NGUYEN TD, NGUYEN TL, et al. Orientation of Human Microprocessor on Primary MicroRNAs. Biochemistry. 2018;58(4):189-198.
[2] REINHART BJ, SLACK FJ, BASSON M, et al. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000;403(6772):901-906.
[3] GILLES ME, SLACK FJ. Let-7 microRNA as a potential therapeutic target with implications for immunotherapy. Expert Opin Ther Targets. 2018; 22(11):929-939.
[4] YAO J, CHENG Y, ZHANG D, et al. Identification of key genes, MicroRNAs and potentially regulated pathways in alcoholic hepatitis by integrative analysis. Gene. 2019;720:144035.
[5] POLAKOVICOVA M, MUSIL P, LACZO E, et al. Circulating MicroRNAs as Potential Biomarkers of Exercise Response. Int J Mol Sci. 2016;7(10): 1553.
[6] CAMERA DM, SMILES WJ, HAWLEY JA. Exercise-induced skeletal muscle signaling pathways and human athletic performance. Free Radic Biol Med. 2016;98:131-143.
[7] GARAVELLI S, DE ROSA V, DE CANDIA P. The multifaceted interface between cytokines and microRNAs: An ancient mechanism to regulate the good and the bad of inflammation. Front Immunol. 2018;9:3012.
[8] GEIGER J, DALGAARD LT. Interplay of mitochondrial metabolism and microRNAs.Cell Mol Life Sci. 2017;74(4):631-646.
[9] RODRIGUES J A, PRíMOLA-GOMES T N, SOARES L L, et al. Physical exercise and regulation of intracellular calcium in cardiomyocytes of hypertensive rats. Arq Bras Cardiol. 2018;111(2):172-179.
[10] YANG F, YOU X, XU T, et al.Screening and function analysis of MicroRNAs involved in exercise preconditioning-attenuating pathological cardiac hypertrophy . Int Heart J. 2018;59(5):1069-1076.
[11] MCCARTHY JJ, ESSER KA. MicroRNA-1 and microRNA-133a expression are decreased during skeletal muscle hypertrophy.J Appl Physiol (1985). 2007;102(1):306-313.
[12] BAGGISH AL, HALE A, WEINER RB, et al.Dynamic regulation of circulating microRNA during acute exhaustive exercise and sustained aerobic exercise training. J Physiol. 2011;589(16):3983-3994.
[13] HORAK M,NOVAK J, BIENERTOVA-VASKU J. Muscle-specific microRNAs in skeletal muscle development. Dev Biol. 2016;410(1):1-13.
[14] BEI Y, TAO L, CRETOIU D, et al. MicroRNAs Mediate Beneficial Effects of Exercise in Heart. Adv Exp Med Biol. 2017;1000:261-280.
[15] FAULKNER JA, LARKIN LM, CLAFLIN DR, et al. Age‐related changes in the structure and function of skeletal muscles. Biogerontology. 2018;19(6):519-536.
[16] AOI W. Frontier impact of microRNAs in skeletal muscle research: a future perspective. Front Physiol. 2015;5:495.
[17] HOU X, TANG Z, LIU H, et al. Discovery of MicroRNAs associated with myogenesis by deep sequencing of serial developmental skeletal muscles in pigs. PloS one. 2012;7(12):e52123.
[18] 马翔,唐成林,吴梦佳,等. microRNA调节肌肉萎缩作用机制的研究进展[J].中国康复理论与实践,2019,25(4):68-72.
[19] RUBIŚ P, TOTOŃ‐ŻURAŃSKA J, WIŚNIOWSKA‐ŚMIAŁEK S, et al. The relationship between myocardial fibrosis and myocardial micro RNA s in dilated cardiomyopathy: A link between mir‐133a and cardiovascular events. J Cell Mol Med. 2018;22(4):2514-2517.
[20] ENDO K, WENG H, NAITO Y, et al. Classification of various muscular tissues using miRNA profiling. Biomed Res. 2013;34(6):289-299.
[21] YUASA K, HAGIWARA Y, ANDO M, et al. MicroRNA-206 is highly expressed in newly formed muscle fibers: implications regarding potential for muscle regeneration and maturation in muscular dystrophy. Cell Struct Funct. 2008;33(2):163-169.
[22] CHEN J F, TAO Y, LI J, et al. microRNA-1 and microRNA-206 regulate skeletal muscle satellite cell proliferation and differentiation by repressing Pax7. J Cell Biol. 2010;190(5):867-879.
[23] CACCHIARELLI D, LEGNINI I, MARTONE J, et al. miRNAs as serum biomarkers for Duchenne muscular dystrophy. EMBO Mol Med. 2011; 3(5):258-265.
[24] RUSSELL AP, WADA S, VERGANI L, et al. Disruption of skeletal muscle mitochondrial network genes and miRNAs in amyotrophic lateral sclerosis. Neurobiol Dis. 2013;49:107-117.
[25] MISSIAGLIA E, SHEPHERD CJ, PATEL S, et al. MicroRNA-206 expression levels correlate with clinical behaviour of rhabdomyosarcomas. Br J Cancer. 2010;102(12):1769-1777.
[26] DONALDSON A, NATANEK SA, LEWIS A, et al. Increased skeletal muscle-specific microRNA in the blood of patients with COPD. Thorax. 2013; 68(12):1140-1149.
[27] RINGHOLM S, BIENSO RS, KIILERICH K, et al. Bed rest reduces metabolic protein content and abolishes exercise-induced mRNA responses in human skeletal muscle. Am J Physiol Endocrinol Metab. 2011;301(4): E649-658.
[28] CHISTIAKOV DA, OREKHOV AN, BOBRYSHEV YV. Cardiac-specific miRNA in cardiogenesis, heart function, and cardiac pathology (with focus on myocardial infarction) . J Mol Cell Cardiol. 2016;94:107-121.
[29] NAKAMURA M, SADOSHIMA J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat Rev Cardiol. 2018;15(7):387-407.
[30] DEWENTER M, VON DER LIETH A, KATUS HA, et al. Calcium Signaling and Transcriptional Regulation in Cardiomyocytes. Circ Res. 2017; 121(8):1000-1020.
[31] ZAGLIA T, CERIOTTI P, CAMPO A, et al. Content of mitochondrial calcium uniporter (MCU) in cardiomyocytes is regulated by microRNA-1 in physiologic and pathologic hypertrophy . Proc Natl Acad Sci U S A. 2017;114(43):E9006-E9015.
[32] DINIZ GP, LINO CA, MORENO CR, et al. MicroRNA-1 overexpression blunts cardiomyocyte hypertrophy elicited by thyroid hormone. J Cell Physiol. 2017;232(12):3360-3368.
[33] LEE S Y, LEE C Y, HAM O, et al. microRNA-133a attenuates cardiomyocyte hypertrophy by targeting PKCdelta and Gq. Mol Cell Biochem. 2018; 439(1-2):105-115.
[34] DINIZ GP, LINO CA, GUEDES EC, et al. Cardiac microRNA-133 is down-regulated in thyroid hormone-mediated cardiac hypertrophy partially via Type 1 Angiotensin II receptor. Basic Res Cardiol. 2015;110(5):49.
[35] WANG D, ZHAI G, JI Y, et al. microRNA-10a Targets T-box 5 to Inhibit the Development of Cardiac Hypertrophy. Int Heart J. 2017;58(1):100-106.
[36] YANG Y, ZHOU Y, CAO Z, et al. miR-155 functions downstream of angiotensin II receptor subtype 1 and calcineurin to regulate cardiac hypertrophy. Exp Ther Med. 2016;12(3):1556-1562.
[37] MATSUSHIMA S, SADOSHIMA J. The role of sirtuins in cardiac disease. Am J Physiol Heart Circ Physiol. 2015;309(9):H1375-1389.
[38] NIE X, FAN J, LI H, et al. miR-217 Promotes Cardiac Hypertrophy and Dysfunction by Targeting PTEN. Mol Ther Nucleic Acids. 2018;12:254-266.
[39] THIENPONT B, ARONSEN JM, ROBINSON EL, et al. The H3K9 dimethyltransferases EHMT1/2 protect against pathological cardiac hypertrophy. J Clin Invest. 2017;127(1):335-348.
[40] SASSI Y, AVRAMOPOULOS P, RAMANUJAM D, et al. Cardiac myocyte miR-29 promotes pathological remodeling of the heart by activating Wnt signaling. Nat Commun. 2017;8(1):1614.
[41] ZHANG S, YIN Z, DAI F F, et al. miR-29a attenuates cardiac hypertrophy through inhibition of PPARdelta expression. J Cell Physiol. 2019;234(8): 13252-13262.
[42] BUENO OF, DE WINDT LJ, LIM HW, et al. The dual-specificity phosphatase MKP-1 limits the cardiac hypertrophic response in vitro and in vivo. Circ Res. 2001;88(1):88-96.
[43] LI R, YAN G, LI Q, et al. MicroRNA-145 protects cardiomyocytes against hydrogen peroxide (H(2)O(2))-induced apoptosis through targeting the mitochondria apoptotic pathway. PLoS One. 2012;7(9):e44907.
[44] WANG X, ZHANG X, REN X P, et al. MicroRNA-494 targeting both proapoptotic and antiapoptotic proteins protects against ischemia/reperfusion-induced cardiac injury . Circulation. 2010;122(13):1308-1318.
[45] ZHAO YC. Effects of exercise training on myocardial mitochondrial miR-499-CaN-Drp-1 apoptotic pathway in mice. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2015;31(3):259-263.
[46] ROCA-ALONSO L, CASTELLANO L, MILLS A, et al. Myocardial MiR-30 downregulation triggered by doxorubicin drives alterations in beta-adrenergic signaling and enhances apoptosis. Cell Death Dis. 2015;6: e1754.
[47] WU H, WANG F, HU S, et al. MiR-20a and miR-106b negatively regulate autophagy induced by leucine deprivation via suppression of ULK1 expression in C2C12 myoblasts. Cell Signal. 2012;24(11):2179-2186.
[48] CHANG Y, YAN W, HE X, et al. miR-375 inhibits autophagy and reduces viability of hepatocellular carcinoma cells under hypoxic conditions. Gastroenterology. 2012;143(1):177-87e8.
[49] ZHAO S, YAO D, CHEN J, et al. MiR-20a promotes cervical cancer proliferation and metastasis in vitro and in vivo. PLoS One. 2015;10(3): e0120905.
[50] ZENG Y, HUO G, MO Y, et al. MIR137 Regulates Starvation-Induced Autophagy by Targeting ATG7. J Mol Neurosci. 2015;56(4): 815-821.
[51] WANG K, LIU CY, ZHOU LY, et al. APF lncRNA regulates autophagy and myocardial infarction by targeting miR-188-3p. Nat Commun. 2015;6: 6779.
[52] XU N, ZHANG J, SHEN C, et al. Cisplatin-induced downregulation of miR-199a-5p increases drug resistance by activating autophagy in HCC cell. Biochem Biophys Res Commun. 2012;423(4):826-831.
[53] XU F, KANG Y, ZHANG H, et al. Akt1-mediated regulation of macrophage polarization in a murine model of Staphylococcus aureus pulmonary infection. J Infect Dis. 2013;208(3):528-538.
[54] ROTHCHILD AC, SISSONS JR, SHAFIANI S, et al. MiR-155-regulated molecular network orchestrates cell fate in the innate and adaptive immune response to Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 2016;113(41):E6172-E6181.
[55] WU XQ, DAI Y, YANG Y, et al. Emerging role of microRNAs in regulating macrophage activation and polarization in immune response and inflammation. Immunology. 2016;148(3):237-248.
[56] KIM SW, RAMASAMY K, BOUAMAR H, et al. MicroRNAs miR-125a and miR-125b constitutively activate the NF-kappaB pathway by targeting the tumor necrosis factor alpha-induced protein 3 (TNFAIP3, A20) . Proc Natl Acad Sci U S A. 2012;109(20):7865-7870.
[57] LIU X, XIAO J, ZHU H, et al. miR-222 is necessary for exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell metabolism. 2015;21(4):584-595.
[58] AOI W, NAITO Y, MIZUSHIMA K, et al.The microRNA miR-696 regulates PGC-1α in mouse skeletal muscle in response to physical activity.Am J Physiol Endocrinol Metab. 2010;298(4):E799-806.
[59] SHARMA M, JUVVUNA P K, KUKRETI H, et al. Mega roles of microRNAs in regulation of skeletal muscle health and disease. Front Physiol. 2014;5:239.
[60] HECKSTEDEN A, LEIDINGER P, BACKES C, et al. miRNAs and sports: tracking training status and potentially confounding diagnoses. J Transl Med. 2016;14(1):219.
[61] BYE A, ROSJO H, ASPENES S T, et al. Circulating microRNAs and aerobic fitness--the HUNT-Study. PLoS One. 2013;8(2):e57496.
[62] ELIA L, CONTU R, QUINTAVALLE M, et al. Reciprocal regulation of microRNA-1 and insulin-like growth factor-1 signal transduction cascade in cardiac and skeletal muscle in physiological and pathological conditions. Circulation. 2009;120(23):2377-2385.
[63] IGAZ I, IGAZ P. Possible role for microRNAs as inter-species mediators of epigenetic information in disease pathogenesis: is the non-coding dark matter of the genome responsible for epigenetic interindividual or interspecies communication?. Med Hypotheses. 2015;84(2):150-154.
[64] VALADI H, EKSTROM K, BOSSIOS A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654-659.
[65] RAYNER KJ, HENNESSY EJ. Extracellular communication via microRNA: lipid particles have a new message. J Lipid Res. 2013;54(5):1174-1181.
[66] LI L, ZHU D, HUANG L, et al. Argonaute 2 complexes selectively protect the circulating microRNAs in cell-secreted microvesicles. PLoS One. 2012;7(10):e46957.
[67] VICKERS KC, PALMISANO BT, SHOUCRI BM, et al. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011;13(4):423-433.
[68] KELLER A, MEESE E. Can circulating miRNAs live up to the promise of being minimal invasive biomarkers in clinical settings?. Wiley Interdiscip Rev RNA. 2016;7(2):148-156.
[69] DE GONZALO-CALVO D, DáVALOS A, FERNáNDEZ-SANJURJO M, et al. Circulating microRNAs as emerging cardiac biomarkers responsive to acute exercise. Int J Cardiol. 2018;264:130-136.
[70] AOI W, ICHIKAWA H, MUNE K, et al. Muscle-enriched microRNA miR-486 decreases in circulation in response to exercise in young men. Front Physiol. 2013;4:80.
[71] ZHANG T, BIRBRAIR A, WANG ZM, et al. Improved knee extensor strength with resistance training associates with muscle specific miRNAs in older adults. Exp Gerontol. 2015;62:7-13.
[72] WARDLE SL, BAILEY ME, KILIKEVICIUS A, et al. Plasma microRNA levels differ between endurance and strength athletes. PLoS One. 2015; 10(4):e0122107.
[73] BAGGISH AL, PARK J, MIN PK, et al. Rapid upregulation and clearance of distinct circulating microRNAs after prolonged aerobic exercise. Appl Physiol (1985). 2014;116(5):522-531.
|