| [1] Wu H,Shen J,Yu X,et al.Two stage management of Cierny-Mader type IV chronic osteomyelitis of the long bones. Injury.2017;48(2):511-518.[2] Badie AA,Arafa MS.One-stage surgery for adult chronic osteomyelitis: concomitant use of antibiotic-loaded calcium sulphate and bone marrow aspirate.Int Orthop.2018:1-10.[3] Wagner C,Obst U,Hänsch GM.Implant-associated posttraumatic osteomyelitis: collateral damage by local host defense? Int J Artif Organs.2005;28(11):1172-1180.[4] Sanders J,Mauffrey C.Long bone osteomyelitis in adults: fundamental concepts and current techniques. Orthopedics. 2013;36(5):368.[5] Li Y,Liu L,Wan P,et al.Biodegradable Mg-Cu alloy implants with antibacterial activity for the treatment of osteomyelitis: In vitro and in vivo evaluations.Biomaterials.2016;106:250-263.[6] Ginebra MP,Espanol M,Maazouz Y,et al.Bioceramics and bone healing.EFORT Open Rev.2018:3(5):173-183.[7] Wang Z,Guo Z,Bai H,et al.Clinical evaluation of β-TCP in the treatment of lacunar bone defects: a prospective, randomized controlled study.Mater Sci Eng C Mater Biol Appl. 2013;33(4): 1894-1899.[8] Gan Y,Dai K,Zhang P,et al.The clinical use of enriched bone marrow stem cells combined with porous beta-tricalcium phosphate in posterior spinal fusion.Biomaterials. 2008; 29(29):3973-3982.[9] Jiang SD,Jiang LS,Dai LY.Surgical treatment of calcaneal fractures with use of beta-tricalcium phosphate ceramic grafting.Foot Ankle Int.2008;29(10):1015-1019.[10] Dai LY, Jiang LS.Anterior cervical fusion with interbody cage containing beta-tricalcium phosphate augmented with plate fixation: a prospective randomized study with 2-year follow-up.Eur Spine J. 2008;17(5):698-705.[11] Yamanaka M,Hara K,Kudo J. Bactericidal actions of a silver ion solution on Escherichia coli, studied by energy-filtering transmission electron microscopy and proteomic analysis. Appl Environ Microbiol.2005;71(11):7589-7593. [12] Angelo T,Carla Renata A,Agnese DA,et al.Antibiofilm activity of a monolayer of silver nanoparticles anchored to an amino-silanized glass surface.Biomaterials.2014;35(6): 1779-1788.[13] Mei S,Wang H,Wang W,et al. Antibacterial effects and biocompatibility of titanium surfaces with graded silver incorporation in titania nanotubes. Biomaterials. 2014;35(14): 4255.[14] 张茹梅,鲁媛媛,王静,等.纳米银凝胶联合乳酸菌阴道胶囊治疗放射性阴道炎的临床分析[J].解放军医药杂志,2018,30(3):91-93.[15] Kumar SSD,Rajendran NK,Houreld NN,et al.Recent advances on silver nanoparticle and biopolymer-based biomaterials for wound healing applications.Int J BiolMacromol. 2018;115:165-175.[16] Ashmore D,Chaudhari A,Barlow B,et al.Evaluation of E. coli inhibition by plain and polymer-coated silver nanoparticles. Rev Inst Med Trop Sao Paulo.2018;60:e18.[17] 管捷,程坚,谢幼专,等.含纳米银颗粒的β-磷酸三钙复合支架的抗菌性和促成骨性的研究[J].生物骨科材料与临床研究, 2018, 15(2):9-13,82-83.[18] 施畅,徐丽明,邵安良.纳米银的毒理学研究现状[J].药物分析杂志, 2013,33(12):2025-2033.[19] 刘晶,孙迎春,曾妃菲.载银磷酸锆可影响293T细胞的生长与增殖[J].中国组织工程研究,2013,17(21):3901-3906.[20] Li Y,Qin T,Ingle T,et al.Differential genotoxicity mechanisms of silver nanoparticles and silver ions.Arch Toxicol. 2017;91(1): 509-519.[21] Jung WK,Koo HC,Kim KW,et al.Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli.Appl Environ Microbiol. 2008; 74(7):2171-2178.[22] Shrivastava S,Bera T,Roy A,et al.Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology.2007;18(22):103-112.[23] 林晓华,黎志超,俞金龙,等.新型复合生物抗菌敷料的抗菌强度、吸湿能力及细胞毒性[J].中国组织工程研究, 2013,17(16): 2905-2912.[24] Sharma VK,Yngard RA,Lin Y.Silver nanoparticles: Green synthesis and their antimicrobial activities.Adv Colloid Interface Sci.2009;145(1-2):83-96.[25] Ciobanu CS, Iconaru SL, Le Coustumer P, et al.Antibacterial activity of silver-doped hydroxyapatite nanoparticles against gram-positive and gram-negative bacteria. Nanoscale Res Lett. 2012;7(1):324.[26] Gopi D,Shinyjoy E,Kavitha L.Synthesis and spectral characterization of silver/magnesium co-substituted hydroxyapatite for biomedical applications.Spectrochim Acta A Mol Biomol Spectrosc. 2014;127:286-291.[27] 汤京龙,王硕,刘丽,等.纳米银颗粒的细胞毒性作用及机制初探[J].北京生物医学工程,2013,32(5):485-489.[28] 孙晋都,张帮勇,李婷竹,等.纳米银的银离子释放及其对细胞的毒性作用[J].环境与职业医学,2017,34(7):636-641.[29] Vr?ek IV,?untar I,Petlevski R,et al. Comparison of in vitro toxicity of silver ions and silver nanoparticles on human hepatoma cells.Environ Toxicol.2016;31(6):679-692.[30] Lee Y, Kim P,Yoon J,et al.Serum kinetics, distribution and excretion of silver in rabbits following 28 days after a single intravenous injection of silver nanoparticles.Nanotoxicology. 2013;7(6):1120-1130. [31] Gaiser BK,Hirn S,Kermanizadeh A,et al.Effects of silver nanoparticles on the liver and hepatocytes in vitro.Toxicol Sci.2013;131(2):537-547.[32] Kim YS,Kim JS,Cho HS,et al.Twenty-eight-day oral toxicity, genotoxicity, and gender-related tissue distribution of silver nanoparticles in Sprague-Dawley rats.Inhal Toxicol. 2008; 20(6):575-583.[33] Korani M,Rezayat SM,Gilani K,et al. Acute and subchronic dermal toxicity of nanosilver in guinea pig.Int J Nanomedicine. 2011;6:855-862. |
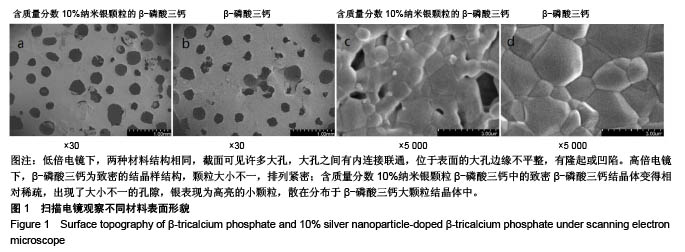
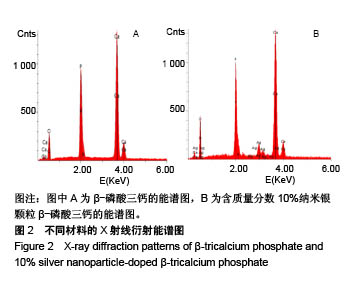
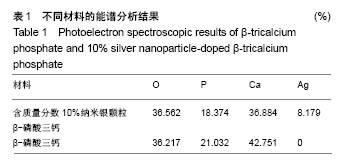
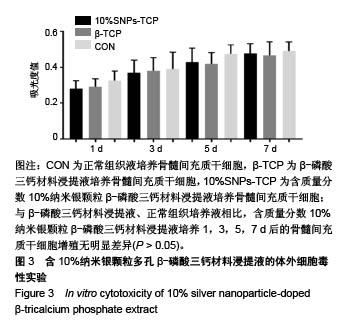
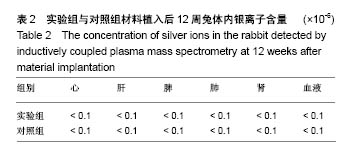
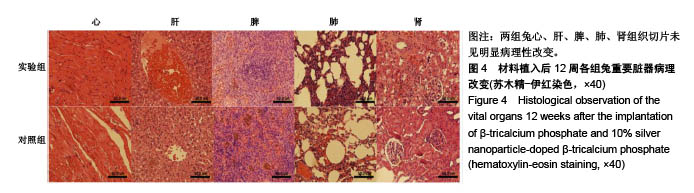
.jpg)
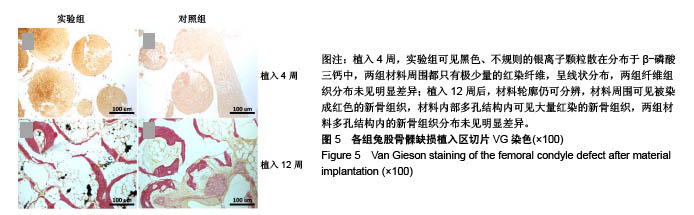
.jpg)