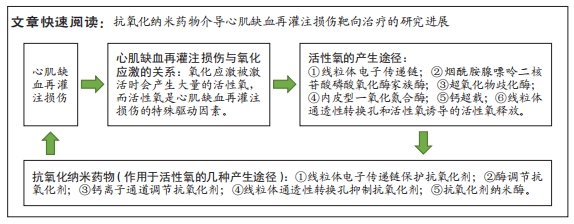
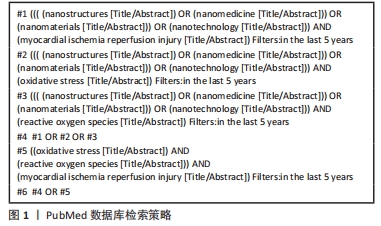
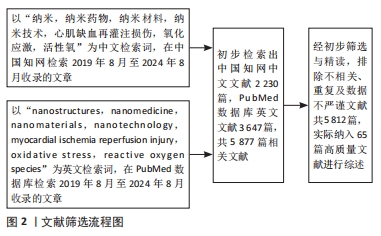
[1] WANG W, HAO H, FAN T, et al. Predictive value of acoustic cardiography for post-PCI early ventricular remodeling in acute myocardial infarction. Sci Rep. 2023;13(1):7192.
[2] MOON BF, IYER SK, HWUANG E, et al. Iron imaging in myocardial infarction reperfusion injury. Nat Commun. 2020;11(1):3273.
[3] ZHANG Y, ZHANG X, CAI B, et al. The long noncoding RNA lncCIRBIL disrupts the nuclear translocation of Bclaf1 alleviating cardiac ischemia-reperfusion injury. Nat Commun. 2021;12(1):522.
[4] LAWTON JS, TAMIS-HOLLAND JE, BANGALORE S, et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(3):e4-e17.
[5] ZHANG S, YAN F, LUAN F, et al. The pathological mechanisms and potential therapeutic drugs for myocardial ischemia reperfusion injury. Phytomedicine. 2024;129:155649.
[6] HE J, HUANG L, SUN K, et al. Oleuropein alleviates myocardial ischemia-reperfusion injury by suppressing oxidative stress and excessive autophagy via TLR4/MAPK signaling pathway. Chin Med. 2024;19(1):59.
[7] ZHANG Z, DALAN R, HU Z, et al. Reactive Oxygen Species Scavenging Nanomedicine for the Treatment of Ischemic Heart Disease. Adv Mater. 2022;34(35):e2202169.
[8] LIU Q, ZOU J, CHEN Z, et al. Current research trends of nanomedicines. Acta Pharm Sin B. 2023;13(11):4391-4416.
[9] AMINU N, BELLO I, UMAR NM, et al. The influence of nanoparticulate drug delivery systems in drug therapy. J Drug Deliv Sci Technol. 2022;60:101961.
[10] GONCHAROV RG, SHARAPOV MG. [Ischemia-Reperfusion Injury: Molecular Mechanisms of Pathogenesis and Methods of Their Correction]. Mol Biol (Mosk). 2023;57(6):1150-1174.
[11] ZHANG X, ZHENG Y, WANG Z, et al. Melatonin as a therapeutic agent for alleviating endothelial dysfunction in cardiovascular diseases: Emphasis on oxidative stress. Biomed Pharmacother. 2023;167:115475.
[12] JUAN CA, PÉREZ DE LA LASTRA JM, PLOU FJ, et al. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int J Mol Sci. 2021;22(9):4642.
[13] AMINU N, BELLO I, UMAR NM. DNA-based nanosystems to generate reactive oxygen species for nanomedicine. CCL. 2024;35(11):109637.
[14] HU Y, LI J, LOU B, et al. The Role of Reactive Oxygen Species in Arsenic Toxicity. Biomolecules. 2020;10(2):240.
[15] MORRESI C, CIANFRUGLIA L, SARTINI D, et al. Effect of High Glucose-Induced Oxidative Stress on Paraoxonase 2 Expression and Activity in Caco-2 Cells. Cells. 2019;8(12):1616.
[16] LI L, WANG Y, GUO R, et al. Ginsenoside Rg3-loaded, reactive oxygen species-responsive polymeric nanoparticles for alleviating myocardial ischemia-reperfusion injury. J Control Release. 2020;317:259-272.
[17] VASAN K, CLUTTER M, FERNANDEZ DUNNE S, et al. Genes Involved in Maintaining Mitochondrial Membrane Potential Upon Electron Transport Chain Disruption. Front Cell Dev Biol. 2022;10:781558.
[18] MAILLOUX RJ. An update on methods and approaches for interrogating mitochondrial reactive oxygen species production. Redox Biol. 2021;45: 102044.
[19] LI X, OU W, XIE M, et al. Nanomedicine-Based Therapeutics for Myocardial Ischemic/Reperfusion Injury. Adv Healthc Mater. 2023;12(20):e2300161.
[20] TABATA FUKUSHIMA C, DANCIL IS, CLARY H, et al. Reactive oxygen species generation by reverse electron transfer at mitochondrial complex I under simulated early reperfusion conditions. Redox Biol. 2024;70:103047.
[21] REUTER S. Mitochondria - More than just batteries for cellular function. Acta Physiol (Oxf). 2022;235(4):e13852.
[22] TABATA FUKUSHIMA C, DANCIL IS, CLARY H, et al. Reactive oxygen species generation by reverse electron transfer at mitochondrial complex I under simulated early reperfusion conditions. Redox Biol. 2024;70:103047.
[23] MATSUSHIMA S, SADOSHIMA J. Yin and Yang of NADPH Oxidases in Myocardial Ischemia-Reperfusion. Antioxidants (Basel). 2022;11(6):1069.
[24] WANG C, ZHU L, YUAN W, et al. Diabetes aggravates myocardial ischaemia reperfusion injury via activating Nox2-related programmed cell death in an AMPK-dependent manner. J Cell Mol Med. 2020;24(12):6670-6679.
[25] HAHNER F, MOLL F, SCHRÖDER K. NADPH oxidases in the differentiation of endothelial cells. Cardiovasc Res. 2020;116(2):262-268.
[26] NABEEBACCUS AA, REUMILLER CM, SHEN J, et al. The regulation of cardiac intermediary metabolism by NADPH oxidases. Cardiovasc Res. 2023;118(17): 3305-3319.
[27] CHIDAMBARAM SB, ANAND N, VARMA SR, et al. Superoxide dismutase and neurological disorders. IBRO Neurosci Rep. 2024;16:373-394.
[28] KUCHARZIK T, ELLUL P, GREUTER T, et al. ECCO Guidelines on the Prevention, Diagnosis, and Management of Infections in Inflammatory Bowel Disease. J Crohns Colitis. 2021;15(6):879-913.
[29] TSUJII Y, NISHIDA T, OSUGI N, et al. Classification and clinical features of adverse drug reactions in patients with ulcerative colitis treated with 5-aminosalicylate acid: a single-center, observational study. Scand J Gastroenterol. 2022;57(2): 190-196.
[30] ZHAO K, TANG J, XIE H, et al. Nicotinamide riboside attenuates myocardial ischemia-reperfusion injury via regulating SIRT3/SOD2 signaling pathway. Biomed Pharmacother. 2024;175:116689.
[31] SONG W, YUAN Y, TAN X, et al. Icariside II induces rapid phosphorylation of endothelial nitric oxide synthase via multiple signaling pathways. PeerJ. 2022;10: e14192.
[32] KUCZERISZKA M, WĄSOWICZ K. Animal models of hypertension: The status of nitric oxide and oxidative stress and the role of the renal medulla. Nitric Oxide. 2022;125-126:40-46.
[33] KELLY KA, HEAPS CL, WU G, et al. Nanoparticle-mediated delivery of tetrahydrobiopterin restores endothelial function in diabetic rats. Nitric Oxide. 2024;148:13-22.
[34] YAO F, ABDEL-RAHMAN AA. Tetrahydrobiopterin paradoxically mediates cardiac oxidative stress and mitigates ethanol-evoked cardiac dysfunction in conscious female rats. Eur J Pharmacol. 2021;909:174406.
[35] RODRIGUES T, PICCIRILLO S, MAGI S, et al. Control of Ca2+ and metabolic homeostasis by the Na+/Ca2+ exchangers (NCXs) in health and disease. Biochem Pharmacol. 2022;203:115163.
[36] ZHANG S, YAN F, LUAN F, et al. The pathological mechanisms and potential therapeutic drugs for myocardial ischemia reperfusion injury. Phytomedicine. 2024;129:155649.
[37] TAKEUCHI A, KIM B, MATSUOKA S. Physiological functions of mitochondrial Na+-Ca2+ exchanger, NCLX, in lymphocytes. Cell Calcium. 2020;85:102114.
[38] BEHERA R, SHARMA V, GREWAL AK, et al. Mechanistic correlation between mitochondrial permeability transition pores and mitochondrial ATP dependent potassium channels in ischemia reperfusion. Biomed Pharmacother. 2023;162: 114599.
[39] MILLARE B, O’ROURKE B, TRAYANOVA N. Hydrogen peroxide diffusion and scavenging shapes mitochondrial network instability and failure by sensitizing ROS-induced ROS release. Sci Rep. 2020;10(1):15758.
[40] BEÀ A, VALERO JG, IRAZOKI A, et al. Cardiac fibroblasts display endurance to ischemia, high ROS control and elevated respiration regulated by the JAK2/STAT pathway. FEBS J. 2022;289(9):2540-2561.
[41] Liu CJ, Yao L, Hu YM, et al. Effect of Quercetin-Loaded Mesoporous Silica Nanoparticles on Myocardial Ischemia-Reperfusion Injury in Rats and Its Mechanism. Int J Nanomedicine. 2021;16:741-752.
[42] ZHANG Z, CHEN Z, YANG L, et al. Platelet Membrane-Encapsulated MSNs Loaded with SS31 Peptide Alleviate Myocardial Ischemia-Reperfusion Injury. J Funct Biomater. 2022;13(4):181.
[43] LI M, REN C. Exploring the protective mechanism of baicalin in treatment of atherosclerosis using endothelial cells deregulation model and network pharmacology. BMC Complement Med Ther. 2022;22(1):257.
[44] CHEN C, LIU W, GU X, et al. Baicalin-loaded Polydopamine modified ZIF-8 NPs inhibits myocardial ischemia/reperfusion injury in rats. J Biomater Sci Polym Ed. 2024;35(12):1863-1878.
[45] ALTSHULER PJ, SCHIAZZA AR, LUO L, et al. Superoxide Dismutase-Loaded Nanoparticles Attenuate Myocardial Ischemia-Reperfusion Injury and Protect Against Chronic Adverse Ventricular Remodeling. Adv Ther (Weinh). 2021;4(6):2100036.
[46] JIANG L, YAO H, LUO X, et al. Polydopamine-Modified Copper-Doped Titanium Dioxide Nanotube Arrays for Copper-Catalyzed Controlled Endogenous Nitric Oxide Release and Improved Re-Endothelialization. ACS Appl Bio Mater. 2020; 3(5):3123-3136.
[47] VIOLA HM, SHAH AA, KRETZMANN JA, et al. A dendronized polymer variant that facilitates safe delivery of a calcium channel antagonist to the heart. Nanomedicine. 2020;29:102264.
[48] GAO T, YANG P, FU D, et al. The protective effect of allicin on myocardial ischemia-reperfusion by inhibition of Ca2+ overload-induced cardiomyocyte apoptosis via the PI3K/GRK2/PLC-γ/IP3R signaling pathway. Aging (Albany NY). 2021;13(15):19643-19656.
[49] LIU S, HE Y, SHI J, et al. Allicin Attenuates Myocardial Ischemia Reperfusion Injury in Rats by Inhibition of Inflammation and Oxidative Stress. Transplant Proc. 2019;51(6):2060-2065.
[50] DENG X, YANG P, GAO T, et al. Allicin attenuates myocardial apoptosis, inflammation and mitochondrial injury during hypoxia-reoxygenation: an in vitro study. BMC Cardiovasc Disord. 2021;21(1):200.
[51] LIU M, YANG P, FU D, et al. Allicin protects against myocardial I/R by accelerating angiogenesis via the miR-19a-3p/PI3K/AKT axis. Aging (Albany NY). 2021;13(19):22843-22855.
[52] XU Q, XIAO Z, YANG Q, et al. Hydrogel-based cardiac repair and regeneration function in the treatment of myocardial infarction. Mater Today Bio. 2024;25: 100978.
[53] Han X, Lu B, Zou D, et al. Allicin-Loaded Intelligent Hydrogel Coating Improving Vascular Implant Performance. ACS Appl Mater Interfaces. 2023;15(32): 38247-38263.
[54] BARROS CDS, LIVRAMENTO JB, MOURO MG, et al. L-Arginine Reduces Nitro-Oxidative Stress in Cultured Cells with Mitochondrial Deficiency. Nutrients. 2021;13(2):534.
[55] LIU X, ZHANG L, JIANG W, et al. In vitro and in vivo evaluation of liposomes modified with polypeptides and red cell membrane as a novel drug delivery system for myocardium targeting. Drug Deliv. 2020;27(1):599-606.
[56] WANG Z, YANG N, HOU Y, et al. L-Arginine-Loaded Gold Nanocages Ameliorate Myocardial Ischemia/Reperfusion Injury by Promoting Nitric Oxide Production and Maintaining Mitochondrial Function. Adv Sci (Weinh). 2023;10(26):e2302123.
[57] GENDRON A, LAN LINH TRAN N, LALOY J, et al. New Nanoparticle Formulation for Cyclosporin A: In Vitro Assessment. Pharmaceutics. 2021;13(1):91.
[58] ZHANG X, SUN Y, YANG R, et al. An injectable mitochondria-targeted nanodrug loaded-hydrogel for restoring mitochondrial function and hierarchically attenuating oxidative stress to reduce myocardial ischemia-reperfusion injury. Biomaterials. 2022;287:121656.
[59] ZHANG Y, KHALIQUE A, DU X, et al. Biomimetic Design of Mitochondria-Targeted Hybrid Nanozymes as Superoxide Scavengers. Adv Mater. 2021;33(9):e2006570.
[60] XIANG K, WU H, LIU Y, et al. MOF-derived bimetallic nanozyme to catalyze ROS scavenging for protection of myocardial injury. Theranostics. 2023;13(8):2721-2733.
[61] YE T, CHEN C, WANG D, et al. Protective effects of Pt-N-C single-atom nanozymes against myocardial ischemia-reperfusion injury. Nat Commun. 2024;15(1):1682.
[62] SANITÀ G, CARRESE B, LAMBERTI A, et al. Nanoparticle Surface Functionalization: How to Improve Biocompatibility and Cellular Internalization. Front Mol Biosci. 2020;7:587012.
[63] SIAFAKA PI, BÜLBÜL EÖ, MILIOTOU AN, et al.Nano-based carriers for pulmonary drug delivery: A review on the available drug delivery applications and toxicity issues. J Drug Deliv Sci Technol. 2024;92: 1773-2247.
[64] TOUQEER SI, JAHAN N, ABBAS N, et al. Formulation and Process Optimization of Rauvolfia serpentina Nanosuspension by HPMC and In Vitro Evaluation of ACE Inhibitory Potential. J Funct Biomater. 2022;13(4):268.
[65] GARG P, PAREEK S, KULKARNI P, et al. Nanoengineering Solutions for Cancer Therapy: Bridging the Gap between Clinical Practice and Translational Research. J Clin Med. 2024;13(12):3466. |