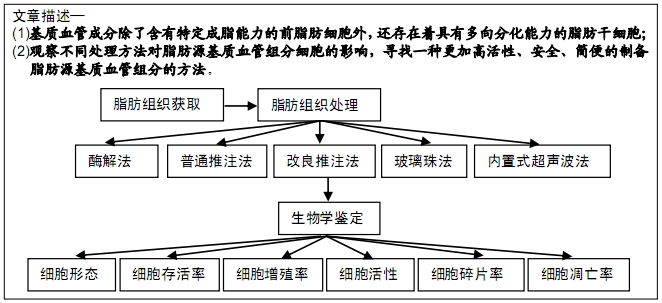
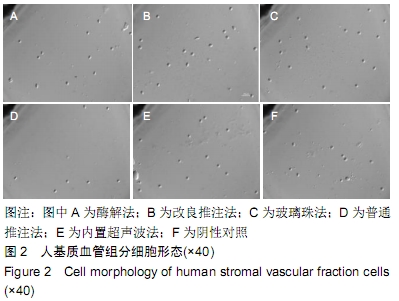
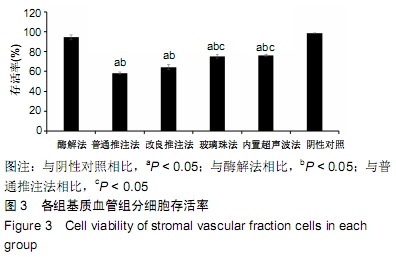
[1] CHUNG MT, ZIMMERMANN AS, PAIK KJ, et al. Isolation of human adipose-derived stromal cells using laser-assisted liposuction and their therapeutic potential in regenerative medicine. Stem Cells Transl Med. 2013;2(10):808-817.
[2] ZUK PA.The adipose-derived stem cell: looking back and looking ahead. Mol Biol Cell. 2010;21(11):1783-1787.
[3] ZUK PA, ZHU M, ASHJIAN P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13(12): 4279-4295.
[4] 张仔昂,鲁峰.脂肪组织工程中生物支架材料的研究进展[J].中国美容整形外科志,2018,29(4):249-251,262.
[5] PREMARATNE GU, MA LP, FUJITA M, et al. Stromal vascular fraction transplantation as an alternative therapy for ischemic heart failure: anti-inflammatory role. J Cardiothorac Surg. 2011; 6:43.
[6] ATALAY S, CORUH A, DENIZ K. Stromal vascular fraction improves deep partial thickness burn wound healing. Burns. 2014;40(7): 1375-1383.
[7] 侯崇超,周传德. SVF-gel联合自体颗粒脂肪在外生殖器整形中的临床应用[J].中国美容整形外科杂志, 2019;30(3):179-181,195.
[8] 张杰,丁权威,王人彦.脂肪组织中的血管基质成分在骨科中的应用研究进展[J].现代实用医学,2017,29(8):1116-1117.
[9] BATEMAN ME, STRONG AL, GIMBLE JM, et al. Concise Review: Using Fat to Fight Disease: A Systematic Review of Nonhomologous Adipose-Derived Stromal/Stem Cell Therapies. Stem Cells. 2018;36(9):1311-1328.
[10] BAGLIONI S, FRANCALANCI M, SQUECCO R, et al. Characterization of human adult stem-cell populations isolated from visceral and subcutaneous adipose tissue. FASEB J. 2009; 23(10):3494-3505.
[11] CHAU TA, MCCULLY ML, BRINTNELL W, et al. Toll-like receptor 2 ligands on the staphylococcal cell wall downregulate superantigen-induced T cell activation and prevent toxic shock syndrome. Nat Med. 2009;15(6):641-648.
[12] ZUK PA, ZHU M, MIZUNO H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211-228.
[13] BUNNELL BA, FLAAT M, GAGLIARDI C, et al. Adipose-derived stem cells: isolation, expansion and differentiation. Methods. 2008;45(2): 115-120.
[14] MOUW JK, OU G, WEAVER VM. Extracellular matrix assembly: a multiscale deconstruction. Nat Rev Mol Cell Biol. 2014;15(12): 771-785.
[15] OSINGA R, MENZI NR, TCHANG LA, et al. Effects of intersyringe processing on adipose tissue and its cellular components: implications in autologous fat grafting. Plast Reconstr Surg. 2015; 135(6):1618-1628.
[16] YAO Y, DONG Z, LIAO Y, et al. Adipose Extracellular Matrix/Stromal Vascular Fraction Gel: A Novel Adipose Tissue-Derived Injectable for Stem Cell Therapy. Plast Reconstr Surg. 2017;139(4):867-879.
[17] 陈信恺,任婧,袁艺,等.脂肪干细胞细胞在面部容量填充和年轻化中的应用[J].中国美容整形外科杂志,2018,29(9):542-544.
[18] BOSCH J, HOUBEN AP, RADKE TF, et al. Distinct differentiation potential of "MSC" derived from cord blood and umbilical cord: are cord-derived cells true mesenchymal stromal cells. Stem Cells Dev. 2012;21(11):1977-1988.
[19] MIDWOOD KS, WILLIAMS LV, Schwarzbauer JE. Tissue repair and the dynamics of the extracellular matrix. Int J Biochem Cell Biol. 2004;36(6):1031-1037.
[20] SHAH FS, WU X, DIETRICH M, et al. A non-enzymatic method for isolating human adipose tissue-derived stromal stem cells. Cytotherapy. 2013;15(8):979-985.
[21] TONNARD P, VERPAELE A, PEETERS G, et al. Nanofat grafting: basic research and clinical applications. Plast Reconstr Surg. 2013;132(4):1017-1026.
[22] VAN DONGEN JA, STEVENS HP, HARMSEN MC, et al. Mechanical Micronization of Lipoaspirates: Squeeze and Emulsification Techniques. Plast Reconstr Surg. 2017;139(6): 1369e-1370e.
[23] GARDIN C, BRESSAN E, FERRONI L, et al. In vitro concurrent endothelial and osteogenic commitment of adipose-derived stem cells and their genomical analyses through comparative genomic hybridization array: novel strategies to increase the successful engraftment of tissue-engineered bone grafts. Stem Cells Dev. 2012;21(5):767-777.
[24] OSINGA R, MENZI NR, TCHANG LA, et al. Effects of intersyringe processing on adipose tissue and its cellular components: implications in autologous fat grafting. Plast Reconstr Surg. 2015; 135(6):1618-1628.
[25] ZIMMERLIN L, DONNENBERG VS, PFEIFER ME, et al. Stromal vascular progenitors in adult human adipose tissue. Cytometry A. 2010;77(1):22-30.
[26] 柳大烈,王晋煌.医疗美容中的吸脂术[J].中国医疗美容, 2012(3): 57-58.
[27] PU LLQ. Fat Grafting for Facial Rejuvenation and Contouring: A Rationalized Approach. Ann Plast Surg. 2018;81(6S Suppl 1): S102-S108.
[28] BANAS A. Purification of adipose tissue mesenchymal stem cells and differentiation toward hepatic-like cells. Methods Mol Biol. 2012;826: 61-72.
[29] MOUW JK, OU G, WEAVER VM. Extracellular matrix assembly: a multiscale deconstruction. Nat Rev Mol Cell Biol. 2014;15(12): 771-785.
[30] 冯春朝,卢沛伶,黄婧,等.改良推注方法制备脂肪移植SVF胶的实验研究[J].中国美容整形外科杂志,2019,30(6):338-341.
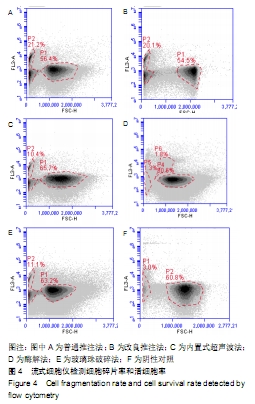
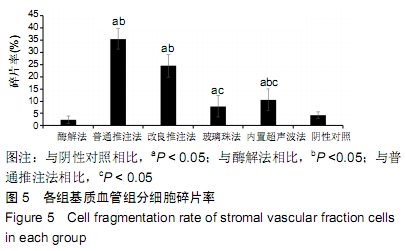
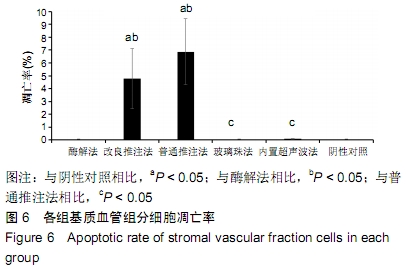
[31] 魏嘉田,卢沛伶,黄婧,等.不同非接触式超声波处理获得脂肪源基质血管组分细胞方法的比较[J].中国组织工程研究,2019,23(33): 5347-5352.
[32] YOSHIMURA K, SHIGEURA T, MATSUMOTO D, et al. Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J Cell Physiol. 2006;208(1):64-76.
[33] 宋正海.关于血细胞计数板的解读[J].生物学教学,2018,43(10): 68-69.
[34] 袁清波.细胞分类计数方法的研究[D].成都:电子科技大学, 2017.
[35] WATANABE M, UEHARA Y, YAMASHITA N, et al. Evaluation of a novel flow cytometry-based system for the detection of circulating tumor cells. Cancer Research. 2012;72(8 Supplement):1735.
[36] HORI G, SAREH A, ABBAS SL, et al. Influence of adipose derived mesenchymal stem cells on the effective inflammatory factors of diabetic wound healing in animal models. Journal of Mazandaran University of Medical Sciences. 2017;27(148):12-21.
[37] BHAT IA, T B S, SOMAL A, et al. An allogenic therapeutic strategy for canine spinal cord injury using mesenchymal stem cells. J Cell Physiol. 2019;234(3):2705-2718.
[38] EBISAWA K, KATO R, OKADA M, et al. Cell therapy for facial anti-aging. Med J Malaysia. 2008;63 Suppl A:41.
[39] TABATABAEI QOMI R, SHEYKHHASAN M. Adipose-derived stromal cell in regenerative medicine: A review. World J Stem Cells. 2017;9(8): 107-117.
[40] JANUS JR, JACKSON RS, LEES KA, et al. Human Adipose-Derived Mesenchymal Stem Cells for Osseous Rehabilitation of Induced Osteoradionecrosis: A Rodent Model. Otolaryngol Head Neck Surg. 2017;156(4):616-621.
[41] HUANG SJ, FU RH, SHYU WC, et al. Adipose-derived stem cells: isolation, characterization, and differentiation potential. Cell Transplant. 2013;22(4):701-709.
[42] LIN CS, XIN ZC, DENG CH, et al. Defining adipose tissue-derived stem cells in tissue and in culture. Histol Histopathol. 2010;25(6): 807-815.
[43] MELIGA E, STREM BM, DUCKERS HJ, et al. Adipose-derived cells. Cell Transplant. 2007;16(9):963-970.
[44] RAMAKRISHNAN VM, BOYD NL. The Adipose Stromal Vascular Fraction as a Complex Cellular Source for Tissue Engineering Applications. Tissue Eng Part B Rev. 2018;24(4):289-299.
[45] BORA P, MAJUMDAR AS. Adipose tissue-derived stromal vascular fraction in regenerative medicine: a brief review on biology and translation. Stem Cell Res Ther. 2017;8(1):145.
[46] MENZI N, OSINGA R, TODOROV A, et al. Wet milling of large quantities of human excision adipose tissue for the isolation of stromal vascular fraction cells. Cytotechnology. 2018;70(2): 807-817.
[47] PRIGLINGER E, SCHUH CMAP, STEFFENHAGEN C, et al. Improvement of adipose tissue-derived cells by low-energy extracorporeal shock wave therapy. Cytotherapy. 2017;19(9): 1079-1095.
[48] SCHENDEL SA. Autologous Adipose-Derived Tissue Matrix Part I: Biologic Characteristics. Aesthet Surg J. 2017;37(9):1062-1068.
[49] SCHENDEL SA. Autologous Adipose-Derived Tissue Matrix Part II: Implantation Biology. Aesthet Surg J. 2017;37(9):1069-1074.
|