中国组织工程研究 ›› 2016, Vol. 20 ›› Issue (16): 2376-2383.doi: 10.3969/j.issn.2095-4344.2016.16.013
• 材料生物相容性 material biocompatibility • 上一篇 下一篇
国产多孔钽复合骨形态发生蛋白7植入兔竖脊肌内的生物相容性
张 辉1,王 茜2,陶建峰1,王爱军3,史 伟4,卞育婕2,李琪佳5,王志强4
- 唐山市第二医院,1关节一科,3创伤二科,河北省唐山市 063000;华北理工大学,2基础医学院人体解剖学系,5医学中心实验室,河北省唐山市 063000; 4华北理工大学附属医院骨科,河北省唐山市 063000
Biocompatibility of domestic porous tantalum carrying bone morphogenetic protein 7 in the erector spinae muscle of rabbits
Zhang Hui1, Wang Qian2, Tao Jian-feng1, Wang Ai-jun3, Shi Wei4, Bian Yu-jie2, Li Qi-jia5, Wang Zhi-qiang4
- 1First Department of Joint Surgery, the Second Hospital of Tangshan, Tangshan 063000, Hebei Province, China; 2Department of Anatomy, Basic Medical College of North China University of Science and Technology, Tangshan 063000, Hebei Province, China; 3Second Department of Traumatology, the Second Hospital of Tangshan, Tangshan 063000, Hebei Province, China; 4Department of Orthopaedics, Affiliated Hospital of North China University of Science and Technology, Tangshan 063000, Hebei Province, China; 5Experimental Center of North China University of Science and Technology, Tangshan 063000, Hebei Province, China
摘要:
文章快速阅读:
.jpg)
文题释义:
骨形态发生蛋白7:又称成骨蛋白1,是骨形态发生蛋白家族中的一员,有促进软骨和骨形成的作用,能在体外促进软骨细胞的增殖和软骨细胞外基质合成,同时具备很强的异位成骨作用,可在异位诱导新骨的形成,在骨缺损修复及软骨分化过程中发挥重要作用。骨形态发生蛋白可通过促进间充质细胞向软骨细胞分化、增殖,增加基质合成等途径,修复关节软骨损伤。
多孔钽:弹性模量为2.0-4.0 GPa,介于皮质骨(12-18 GPa)和松质骨(0.1-0.53 GPa)之间,置入骨骼后几乎没有应力遮挡,利于正常的生物学应力传导,可促进多孔钽和宿主骨的整合。同时多孔钽具备良好的空间三维立体结构,孔隙率可达75%-85%,平均孔径在400-600 µm,利于细胞长入,可为细胞、血管、组织代谢产物及营养物质运输提供有利的空间环境。
背景:骨形态发生蛋白7在体内可诱导骨及软骨形成,并可诱导肌肉中和血管周围的间充质细胞分化为软骨和骨细胞,有促进软骨和骨形成的作用。
目的:观察多孔钽复合骨形态发生蛋白7植入兔竖脊肌后,钽-肌肉界面纤维包膜结构、肌肉与小血管向多孔钽内生性生长及异位成骨的能力。
方法:在新西兰大白兔左右两侧竖脊肌内分别植入复合骨形态发生蛋白7的多孔钽片(实验组)和多孔钽片(对照组),植入后2,4,8周,取钽片及其周围0.5 cm肌肉组织,进行扫描电镜、苏木精-伊红染色、Masson染色及硬组织切片观察。
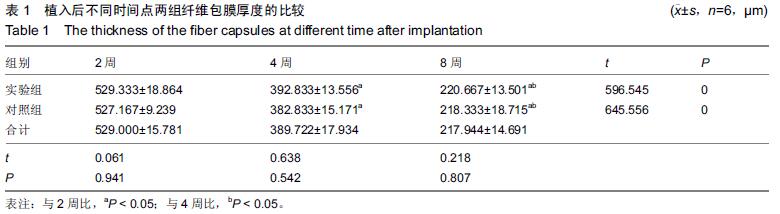
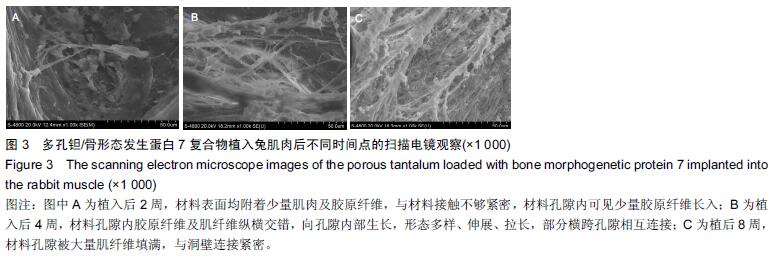
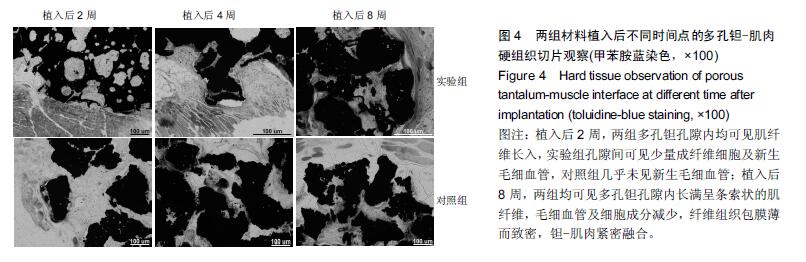
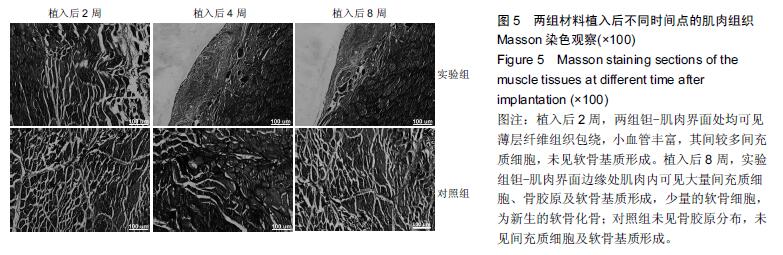
结果与结论:①苏木精-伊红染色:两组材料周围均有纤维性包膜形成,随时间延长,纤维性包膜逐渐由疏松变致密,厚度也逐渐变薄,材料与肌肉交界面无明显炎症反应。两组间纤维包膜厚度比较差异无显著性意义。②扫描电镜:植入2周时,两组多孔钽表面可见少量肌肉及胶原纤维逐渐长入孔隙内部,部分胶原纤维附着于孔壁;植入8周时,多孔钽孔隙内充满了肌腱纤维,纤维与孔壁结合紧密,两组间无明显差异。③硬组织切片:植入2周时,两组多孔钽孔隙均内有少量成纤维细胞及肌纤维长入,实验组材料孔隙内可见有新生小血管长入;植入8周时,两组多孔钽表面和孔隙内均长满呈条索状交 错排列的肌纤维,小血管及细胞成分减少,钽-肌肉紧密融合。④Masson染色:植入8周时,实验组钽-肌肉界面边缘处肌肉内可见大量间充质细胞、骨胶原及软骨基质形成,以及少量新生的软骨化骨,对照组未见软骨化骨。⑤结果表明,复合骨形态发生蛋白7的多孔钽具有良好生物相容性及诱导成骨作用。
中国组织工程研究杂志出版内容重点:生物材料;骨生物材料; 口腔生物材料; 纳米材料; 缓释材料; 材料相容性;组织工程
ORCID: 0000-0002-2962-5069(王志强)
.jpg)